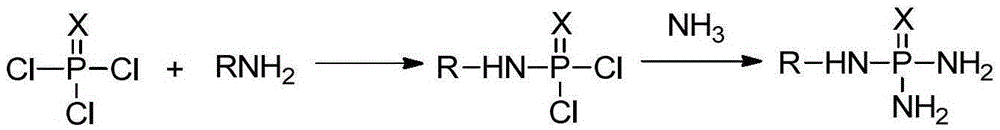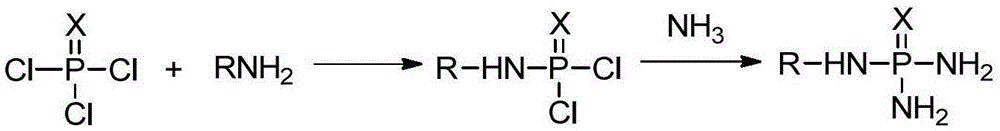Production method of N-alkyl substituted phosphoric triamide
A technology of hydrocarbyl substitution and phosphoric triamide, which is applied in the fields of chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problems of restricting large-scale industrial production, reducing reaction yield and prolonging reaction Time and other issues, to achieve the effect of inhibiting side reactions, increasing reaction yield, and speeding up reaction speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1 (N-n-butyl thiophosphoric triamide)
[0046] Dissolve 25.43 g of phosphorus trichloride in 180 g of ethyl acetate, put it into a four-necked flask equipped with stirring, first add 15.18 grams of triethylamine, and add 11.54 g of n-butylamine dropwise to the ethyl acetate of phosphorus trichloride For the ester solution, the dropping time is maintained at 1 hour, the holding time is 0.5 hours, the reaction temperature is controlled at -30°C, the content of phosphorus trichloride cannot be detected by sampling and analysis, and the reaction is ended to obtain the reaction solution of the first step;
[0047] Open the ammonia gas cylinder, feed ammonia gas, ammonia gas passes through a cold hydrazine, and it can be seen from the outside that liquid ammonia gradually emerges. After a certain amount of liquid ammonia is produced, start the refrigeration device in a four-necked flask equipped with stirring. Maintain the temperature at -45°C, add 12.75g of liquid...
Embodiment 2
[0048] Embodiment 2 (N-n-propyl thiophosphoric triamide)
[0049] Dissolve 25.43 g of phosphorus trichloride in 180 g of ethyl acetate, put it into a four-necked flask equipped with stirring, first add 15.18 grams of triethylamine, and add 8.9 g of n-propylamine dropwise to the ethyl acetate of phosphorus trichloride solution, the dropping time is maintained at 1 hour, the holding time is 0.5 hours, the reaction temperature is controlled at -15°C, the content of phosphorus trichloride cannot be measured by sampling and analysis, the reaction is ended, and the reaction solution of the first step is obtained;
[0050] Put the autoclave into a low-temperature oil bath, keep the temperature in the autoclave at -20°C, close the lid of the autoclave, connect the ammonia gas cylinder to the autoclave, and feed the ammonia gas into the autoclave to maintain the pressure of the autoclave 0.18MPa, the temperature of the autoclave is kept at -20°C, it can be observed that ammonia has bec...
Embodiment 3
[0051] Embodiment 3 (the mixture of N-n-butyl thiophosphoric triamide and N-propyl thiophosphoric triamide)
[0052] Dissolve 25.43g of phosphorus trichloride in 160g of ethyl acetate, put it into a four-neck flask equipped with stirring, first add 15.18g of triethylamine, then add 6.8g of n-butylamine and 3.54g of n-propylamine dropwise to the sulfur trichloride Phosphorous ethyl acetate solution, the dropping time is maintained at 2h, the holding time is 1h, the reaction temperature is controlled at -10°C, the content of phosphorus trichloride cannot be measured by sampling and analysis, the reaction is terminated, and the reaction solution of the first step is obtained;
[0053] Add 13g of liquid ammonia into the reaction kettle, keep the temperature in the reaction kettle at -40°C, and add the pre-cooled reaction solution of the first step dropwise for 1 hour, continue to keep the temperature at -40°C for reaction, and take samples for detection , when N-n-butylaminophosph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com