Tuberculosis immunodiagnosis molecular marker and vaccine use thereof
A technology for tuberculosis and Mycobacterium tuberculosis, applied in the fields of immunology and cell biology, can solve the problems of confusion of lung diseases, high false positive rate, easy false positive rate, etc., and achieve the effect of good coincidence rate and response intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
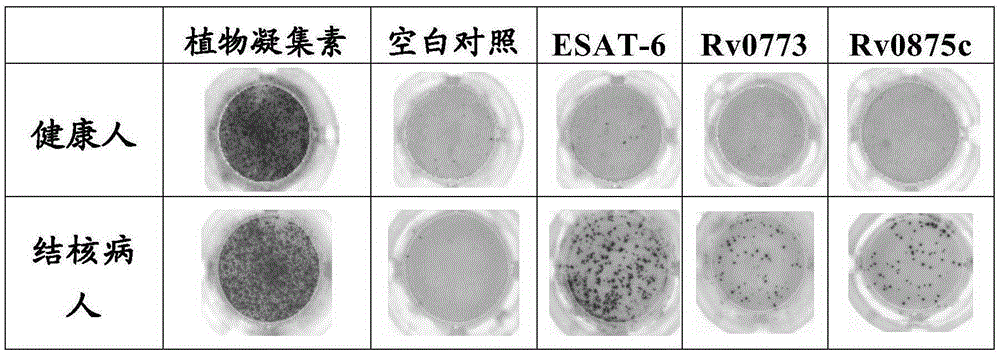
[0071] Example 1: ELISPOT analysis
[0072] Elispot analysis visualizes single-cell secretion products. These assays are exceptionally sensitive because it is captured directly around the secreting cell, and before the surface is diluted, or captured by receptors on adjacent cells, or degraded. Autoimmune detection, organ transplantation, research and development and testing of vaccines and drugs, T cell function research, tumor research, research and detection of infectious diseases, virus research, etc. The specific principle is as follows: cells are stimulated by antigens to produce cytokines, and the cytokine antigens are captured by specific monoclonal antibodies. After cell dissociation, the captured cytokines are bound to a biotin-labeled secondary antibody, followed by alkaline phosphatase-labeled avidin. After incubation with BCIP / NBT substrate, "purple" spots appeared on the PVDF plate, indicating that the cells had produced cytokines, and the results were obtain...
Embodiment 2
[0105] Embodiment 2: Preparation of animal immunity and lymphocyte samples
[0106] In order to verify the results of in vitro cellular immunity tests, identification of the antigenicity of the target protein in vivo is also a very important verification method, so the inventors conducted animal experiments again, and the animal selected was C57BL / 6MarkDoherty, which is a model animal for tuberculosis research [T. Mark Doherty. Tropical Medicine and International Health. New vaccines against tuberculosis. 2004(9): 818-826.].
[0107] 1. Experimental Animals and Grouping
[0108] A total of 50 C57BL / 6 mice (female, 6-8 weeks old) were randomly divided into groups of 6, and the number of the mice was marked by ear cutting. In addition, the remaining 2 mice were used as full negative controls, without immunization, and kept for observation.
[0109] Group 1: PBS, as negative control.
[0110] Group 2: OVA, used as other protein control.
[0111] Group 3: Rv1811, as IFN-γ ne...
Embodiment 3
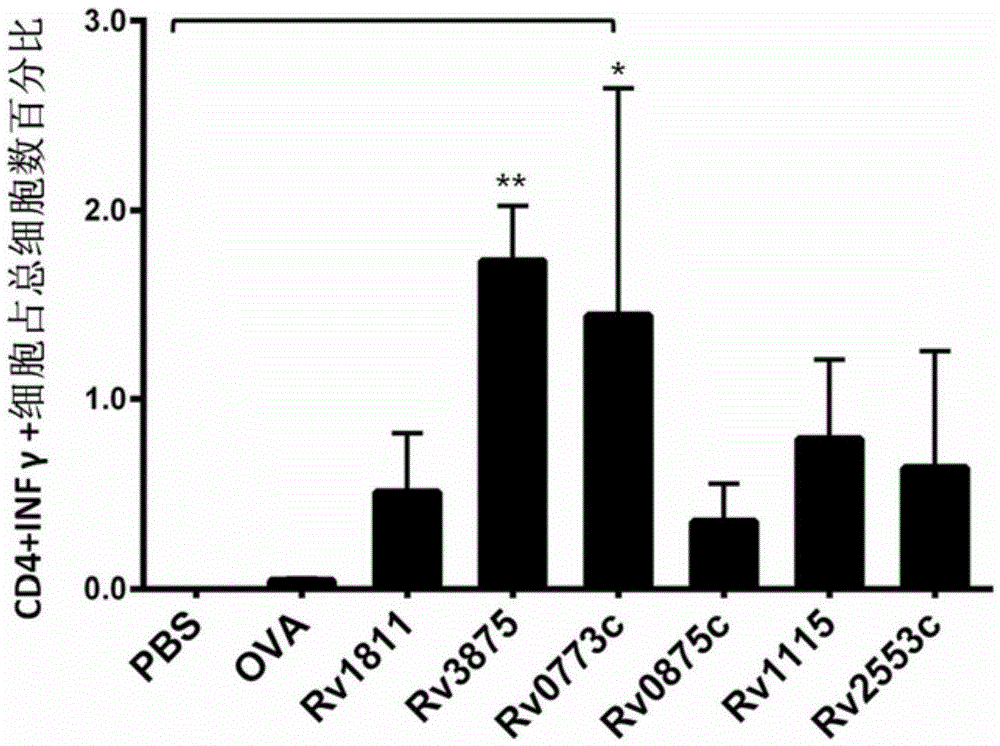
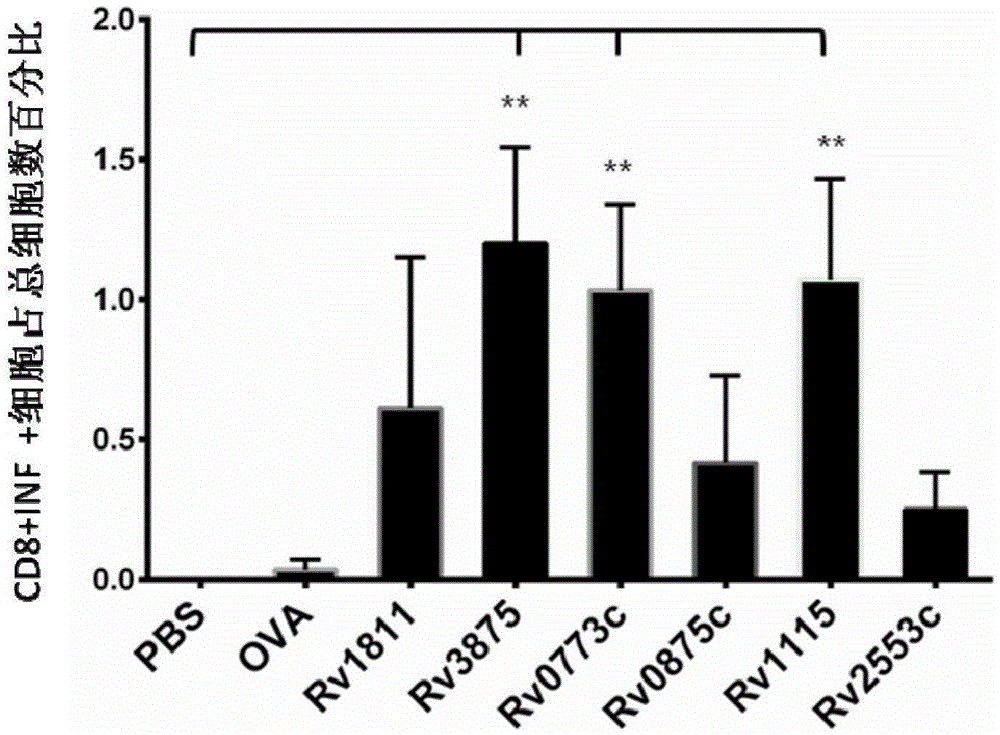
[0127] Example 3: Flow cytometric detection experiment of intracellular factors IFN-γ and IL-2
[0128] 1. Experimental samples and reagents
[0129] Cell samples: lymphocytes from the 8 groups of mouse samples in Example 2 above (8 copies in one batch).
[0130] Kit: CellTrace from invitrogen TM CFSECell Proliferation Kit (C34554).
[0131] 2. Experimental method steps
[0132] (1) A batch of 8 parts of lymphocytes collected in Example 2 was divided into 2×10 6 For dilution, use a 96-well plate, 100 μl per well, and set 3 wells for each lymphocyte. The excess cells were set as a control hole (no stimulator, no staining antibody). A total of 25 holes.
[0133] Wherein, the stimuli are respectively Rv0773c, Rv0875c, Rv1115, Rv2553c, PBS, OVA, negative protein control Rv1811, positive control Rv3875; and the stimuli added to each well correspond to the stimuli used for immunization in the previous Example 2, For example, if the original animal was immunized with Rv0773c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com