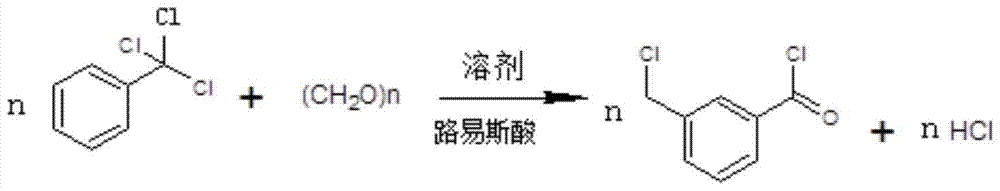
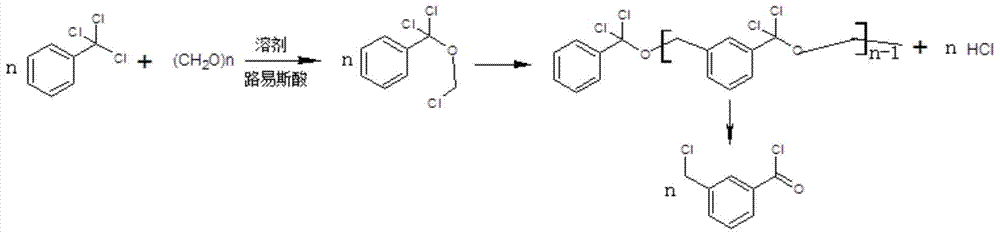
A kind of synthetic method of 3-chloromethylbenzoyl chloride
A technology of chloromethylbenzoyl chloride and a synthetic method, applied in the synthesis of 3-chloromethylbenzoyl chloride and the field of preparation of organic intermediates, can solve the production environment and surrounding environmental hazards, reduce product purity and yield, Increase the difficulty of separation and purification to achieve the effects of improving production safety, improving purity and yield, and reducing inorganic metal ions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
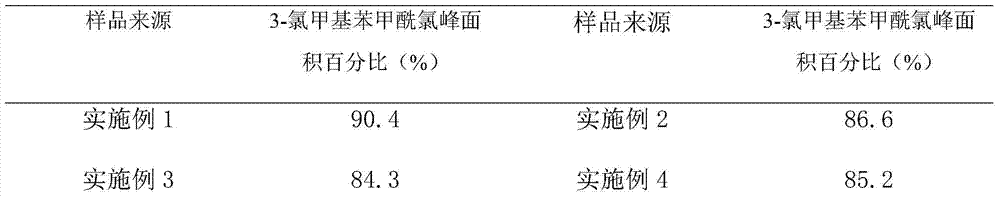
[0024] Put 100ml of dichloromethane into the autoclave, add 0.1mol of trichloromethylbenzene, 0.1mol of paraformaldehyde, 0.05mol of anhydrous aluminum trichloride, fill with nitrogen to 0.4Mpa, and react at 55℃~60℃ for 8h. Then cool down to room temperature, let off the nitrogen, take a sample and carry out GC (Gas Chromatography, gas chromatography) analysis, the area normalization method main peak area accounts for 90.4%, after comparing with the 3-chloromethylbenzoyl chloride reference substance, it is confirmed that The target product is 3-chloromethylbenzoyl chloride.
Embodiment 2
[0026] Put 130ml of chloroform into the autoclave, add 0.1mol of trichloromethylbenzene, 0.15mol of paraformaldehyde, 0.05mol of anhydrous zinc chloride, fill nitrogen to 0.2Mpa, react at 65°C to 70°C for 5h, then cool down to At room temperature, the nitrogen gas was vented, and samples were taken for GC analysis. The area of the main peak accounted for 86.6% by the area normalization method. After comparison with the reference substance of 3-chloromethylbenzoyl chloride, it was confirmed that it was the target product 3-chloromethylbenzoyl chloride.
Embodiment 3
[0028] Put 80ml of carbon tetrachloride into the autoclave, add 0.1mol of trichloromethylbenzene, 0.12mol of paraformaldehyde, 0.02mol of anhydrous ferric chloride, fill with nitrogen to 0.1Mpa, and react at 20℃~25℃ for 20h , then cool down to room temperature, vent nitrogen, take samples for GC analysis, the area normalization method main peak area accounts for 84.3%, after comparing with 3-chloromethylbenzoyl chloride reference substance, it is confirmed that it is the target product 3-chloromethyl Benzoyl chloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com