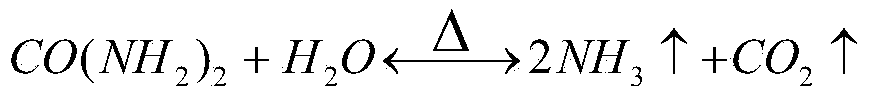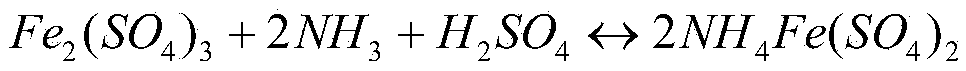Method for preparing iron oxide red from jarosite slag
A technology of jarosite slag and iron red, which is applied in the field of hydrometallurgical comprehensive recovery, can solve the problems of high energy consumption of jarosite slag, long-term stacking of jarosite slag to pollute the environment, and high load requirements of metallurgical equipment, so as to improve the The effect of leaching rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Taking the hydrometallurgical jarosite slag from a Chifeng smelter in Inner Mongolia as the raw material, its elemental composition is shown in Table 1.
[0031] Table 1 The main elements of raw materials
[0032] element
Fe
Zn
Pb
Cu
Cd
Ag
Content w / %
25.9
3.6
1.7
0.22
0.097
518.5
element
S
Mn
Si acid
As
Na
K
Content w / %
10.1
0.92
1.3
0.77
0.61
0.23
[0033] Note: The unit of silver content is g / t
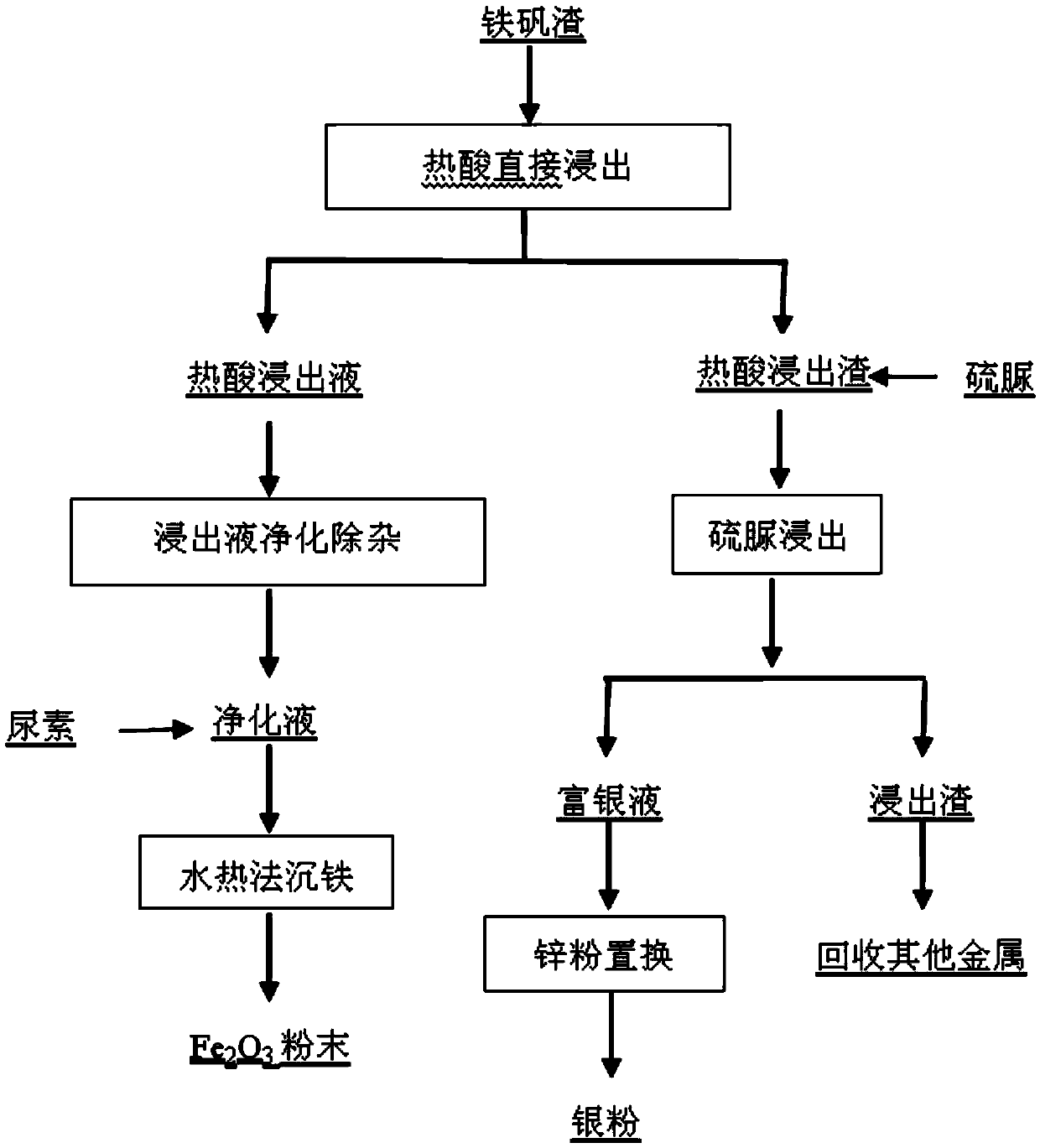
[0034] (1) Weigh 400g of jarosite slag, according to volume / mass ratio (mL / g) 2:1, the amount of sulfuric acid is 1.5 times of the theoretical amount, react at a temperature of 60 ° C for 2h, and filter to obtain the hot acid leaching filtrate and heat acid leaching residue;
[0035] (2) The hot acid leaching solution is replaced by iron powder to remove copper, and sulfide to remove lead, cadmium, arsenic, H 2 O 2 Oxidation to obtain a pur...
Embodiment 2
[0041] Taking the hydrometallurgical jarosite slag from a Chifeng smelter in Inner Mongolia as the raw material, its elemental composition is shown in Table 1.
[0042] (1) Weigh 400g of jarosite slag in, by volume / mass ratio (mL / g) 4:1, the amount of sulfuric acid is 1.0 times of the theoretical amount, react 4h at a temperature of 80 ° C, filter the hot acid leaching filtrate and hot acid leaching residue;
[0043] (2) The hot acid leaching solution is replaced by iron powder to remove copper, and sulfide to remove lead, cadmium, arsenic, H 2 O 2 Oxidation to obtain a purification solution, which contains 103.0g / L of iron;
[0044] (3) the purifying solution is adjusted to pH 1.5 in the milk of lime, adding 1.5 times of urea of the theoretical amount, and the reaction time is 2h under the condition of temperature 180 ℃, and the product iron red is obtained;
[0045] (4) under the condition that the hot acid leaching residue is liquid-solid ratio (mL / g) 10:1, the thioure...
Embodiment 3
[0049] Taking the hydrometallurgical jarosite slag from a Chifeng smelter in Inner Mongolia as the raw material, its elemental composition is shown in Table 1.
[0050] (1) Weigh 400g jarosite slag in, by volume / mass ratio (mL / g) 6:1, the amount of sulfuric acid is 1.8 times of the theoretical amount, react 1h at a temperature of 100 ° C, filter to obtain hot acid leaching filtrate and hot acid leaching residue;
[0051] (2) The hot acid leaching solution is replaced by iron powder to remove copper, and sulfide to remove lead, cadmium, arsenic, H 2 O 2 Oxidation to obtain a purified solution, the purified solution contains 118.6g / L of iron;
[0052] (3) the purifying solution is adjusted to pH 0.5 in the milk of lime, adding 2 times of urea of the theoretical amount, and the reaction time is 3h under the condition of temperature 220 ℃ to obtain the product iron red;
[0053] (4) reacting the hot acid leaching residue at a liquid-solid ratio (mL / g) of 12:1, a thiourea conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com