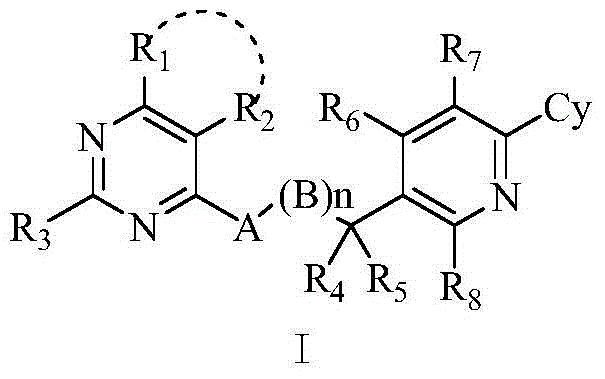
Substituted aryl pyridine compounds and use thereof
An arylpyridine and compound technology, which is applied in the field of agricultural bactericidal, insecticide, and acaricide, and can solve problems such as unreported structural compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0382] Example 1: Preparation of 4,5-dichlorothieno[2,3-d]pyrimidine
[0383]
[0384] Take 2-amino-3-cyano-4-oxyl-4,5-dihydrothiophene and 250mL phosphorus oxychloride (POCl 3 ) into the reaction flask, slowly add 38mL N,N-dimethylformamide (DMF) dropwise at room temperature, and the dropwise addition is completed in about 30 minutes. React at room temperature for 1 hour, then raise the temperature to 75°C for 3 hours. After cooling down to room temperature, the reaction solution was poured into crushed ice and filtered to obtain 89.1 g of a dark gray solid with a yield of 86.9% and a melting point of 160-161°C.
Embodiment 2
[0385] Embodiment 2: Preparation of 2-methyl-6-(4-chlorophenyl)-3-pyridineethylamine
[0386] 1) Preparation of 3-dimethylamino-1-(4-chlorophenyl)-2-propene-one
[0387]
[0388] Put 40.0g (0.26mol) of p-chloroacetophenone into a 500mL single-necked bottle, add 200mL of N,N-dimethylformaldehyde into it, raise the temperature to reflux, and react for 7h. After the completion of the reaction as monitored by TLC, cool to room temperature, distill off the methanol and excess N,N-dimethylformaldehyde generated by the reaction under reduced pressure, a large amount of yellow solid precipitates after cooling, filter with suction, and wash the filter cake with petroleum ether for 2-3 Second, air drying and vacuum drying (70-80° C.) gave 43 g of a yellow solid with a purity of 98.2% and a yield of 79.3%.
[0389] 2) Preparation of ethyl 2-methyl-6-(4-chlorophenyl)nicotinate
[0390]
[0391] Put 24.0g (0.18mol) of ethyl acetoacetate into a 500mL three-neck flask, add 100mL of a...
Embodiment 3
[0404] Embodiment 3: Preparation of 2-methyl-6-(4-chlorophenyl)-3-pyridylbenzylamine
[0405] 1) Preparation of IV-4-1
[0406]
[0407] Put 15.6g (0.062mol) of 2-methyl-3-(chloromethyl)-6-(4-chlorophenyl)-pyridine into a 500mL three-necked flask, and add 200mL of N,N-dimethylformaldehyde Amide, then add 12.0g (0.062mol) phthalimide potassium salt to it, raise the temperature to 50°C, react for 2h, after the reaction is monitored by TLC, cool to room temperature, pour the reaction solution into water, there is a white solid Precipitate, filter with suction, and air-dry to obtain 20.0 g of white solid, purity: 96.2%, yield: 89.1%.
[0408] 2) Preparation of 2-methyl-6-(4-chlorophenyl)-3-pyridylbenzylamine
[0409]
[0410] Put 20.0g (0.055mol) of M-16 into a 500mL three-neck flask, add 200mL of ethanol to it, and then dropwise add 8.0g (0.17mol) of hydrazine hydrate to it, stir at room temperature for a period of time, then gradually raise the temperature to reflux for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com