Targeted fluorescent anti-cancer drug and preparation method thereof
An anticancer drug and fluorescent technology, which is applied in the field of targeted and fluorescent anticancer drugs and their preparation, can solve the damage of tissues and organs, difficult in situ real-time evaluation of tumor changes of anticancer drugs, and normal organ cells. Injury and other problems, to reduce toxic side effects, achieve early tumor diagnosis, strong targeting and fluorescence effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
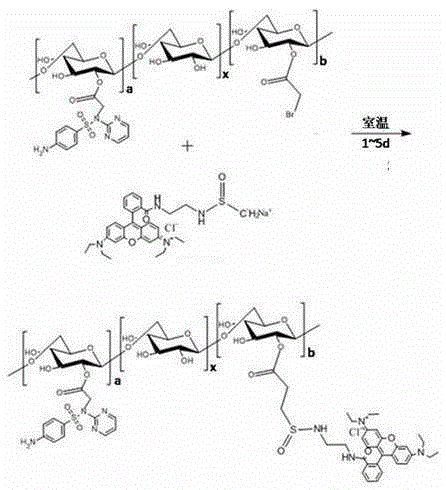
[0083] Step 1, synthesis of bromoacetyl bromide dextran:
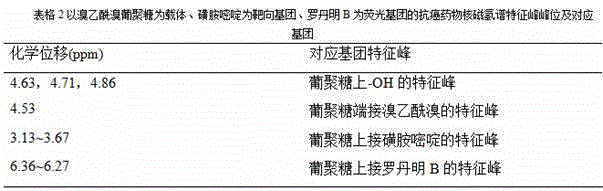
[0084] Dissolve β-glucan 20,000, pyridine and bromoacetyl bromide in dimethyl sulfoxide at a molar ratio of 1:1:0.6, and the molar concentration of β-glucan 20,000 in dimethyl sulfoxide is 0.1 ~1mol / L, react at room temperature for 1~5d, then precipitate the reaction solution with absolute ethanol, filter with suction to obtain a solid, and after drying, dissolve the product in deionized water, dialyze by secondary distillation, and dry under vacuum at 40~80°C to obtain bromine Acetyl bromide dextran.
[0085] Step 2, preparation of sodium sulfadiazine:
[0086] Dissolve sulfadiazine and sodium hydroxide in deionized water at a molar ratio of 1:1, the molar concentration of sulfadiazine and sodium hydroxide in deionized water is 0.1mol / L~1mol / L, react at room temperature for 10~48h, and then The reaction solution was poured into an appropriate amount of absolute ethanol while stirring, and a white precipitate was for...
Embodiment 2
[0098] According to the method described in Example 1, bromoacetyl bromide dextran was prepared, except that 100,000 β-glucan, pyridine and bromoacetyl bromide were used in a molar ratio of 1:1:0.5.
[0099] The synthetic process of step 4 is as follows:
[0100] Bromoacetyl bromide dextran, tetrabutylammonium hydroxide and sodium sulfadiazine were dissolved in dimethyl sulfoxide solution at a molar ratio of 1:0.1:0.3, and the bromoacetyl bromide dextran in dimethyl sulfoxide solution The molar concentration is 0.5mol / L, react at room temperature for 1-5 days, then precipitate with absolute ethanol, filter with suction, dry, dialyze by secondary distillation, and dry in vacuum to obtain bromoacetyl bromide dextran grafted with sulfadiazine;
[0101] The bromoacetyl bromide dextran grafted with sulfadiazine and the ethylenediamine rhodamine B grafted with methanesulfonyl carbanion were mixed according to the molar ratio of 1:0.2 and dissolved in dimethyl sulfoxide solution, and...
Embodiment 3
[0103] Prepare bromoacetyl bromide dextran and sulfadiazine sodium according to the method described in Example 1, the difference is that the molar concentration of rhodamine B in step 3 and ethylenediamine in dehydrated alcohol is 0.1mol / L~ 0.15mol / L, the molar ratio of dimethyl sulfoxide to sodium hydride is 5:1.
[0104] The synthetic process of step 4 is as follows:
[0105] Bromoacetyl bromide dextran, tetrabutylammonium hydroxide and sulfadiazine sodium are dissolved in dimethyl sulfoxide solution according to the molar ratio of 1:0.1:0.1, and the bromoacetyl bromide dextran in dimethyl sulfoxide solution The molar concentration is 0.1mol / L, react at room temperature for 1~5 days, then precipitate with absolute ethanol, filter with suction, dry, dialyze by secondary distillation, and dry in vacuum to obtain bromoacetyl bromide dextran grafted with sulfadiazine;
[0106] The bromoacetyl bromide dextran grafted with sulfadiazine and the ethylenediamine rhodamine B grafted...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com