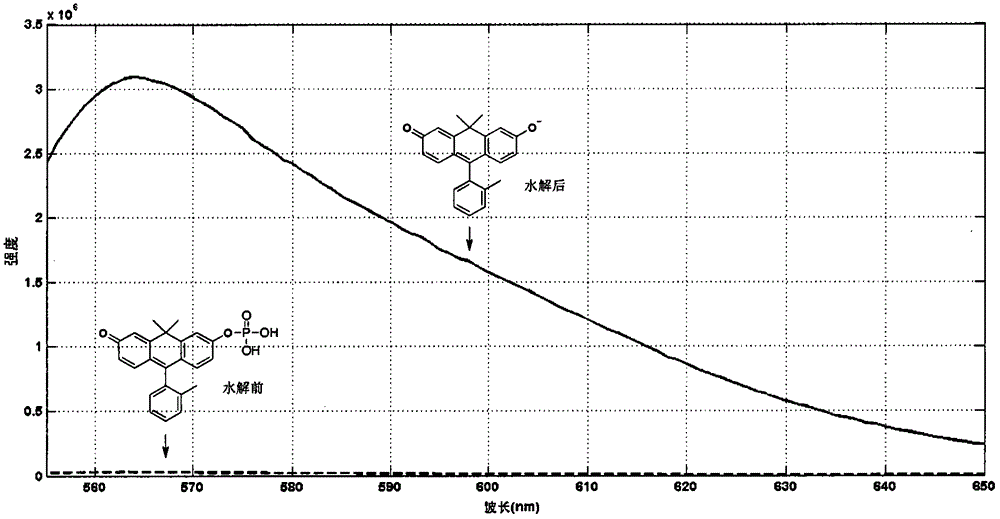
Anthracene fluorescent dye synthesis and application
A fluorescent dye, C1-C6 technology, used in anthracene dyes, organic dyes, luminescent materials, etc., can solve the problems of unstable intermediates, unfavorable large-scale synthesis and derivatization, and achieve high quantum yield, wide biological Application prospects, the effect of high absorption coefficient
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Synthesis of anthracene-structured fluorescein "Beijing Orange":
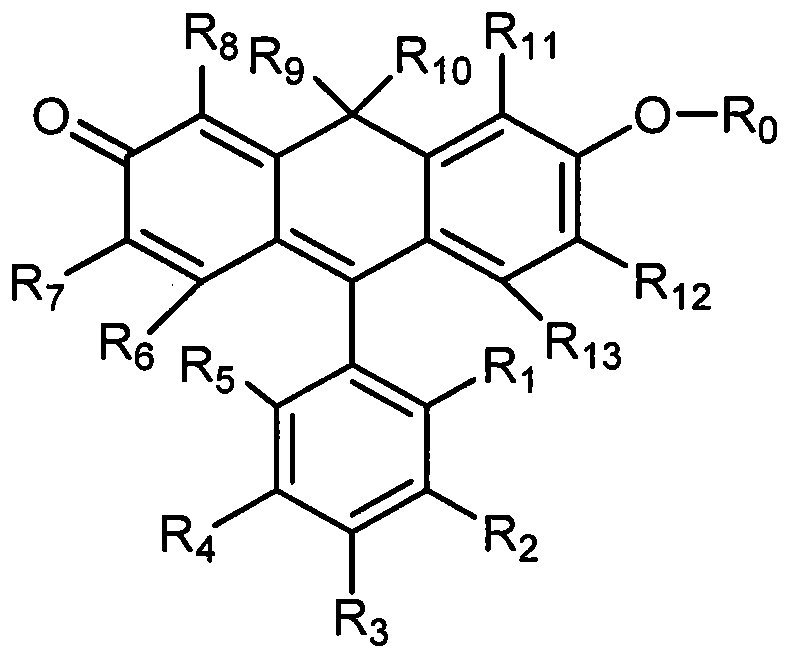
[0039] In the general formula (1), R 1 = Me,R 0 = H, R 2-8,11-13 =H, the synthetic route is as follows
[0040]
[0041] Reagents and reaction conditions: i) THF, -40°C; ii) DCM, PCC, diatomaceous earth; iii) ZnMe 2 , TiCl 4 , DCM-40°C; iv) t-Buli, THF; o-tolualdehyde; v) DCM, PCC; vi) BBr 3 ;MeSO 3 h
[0042] 1. Synthesis of (2-bromo-5-methoxyphenyl)-3-methoxybenzyl alcohol 1a
[0043]
[0044] In a 250ml dry round bottom bottle equipped with a constant pressure dropping funnel, cool the 80ml dry tetrahydrofuran solution of m-methoxyphenylmagnesium chloride to -40°C, and dissolve 3-methoxy-o-bromobenzaldehyde under the protection of argon. (10.8g) of 20ml dry tetrahydrofuran solution was added dropwise into the reaction flask through a constant pressure dropping funnel, kept at -40°C and stirred for 2-6 hours, and the reaction was monitored by TLC. After the disappearance of the raw materi...
Embodiment 2
[0067] Synthesis of Fluorescent Producible Phosphatase Substrate Molecules with Anthracene Structure 2
[0068]
[0069] Add 300 mg of compound 1 to the dry reaction bottle, then add 15 mL of anhydrous dry dichloromethane, and stir. To this suspension was added the proton sponge and stirred for 10 minutes until the mixture was dissolved. Cool to -20~0°C, add 250uL phosphorus oxychloride dropwise, and keep the reaction at low temperature for 0.5~2h. Add 30 mL of sodium phosphate buffer and continue stirring for 1 hour. The resulting solution was separated, the aqueous phase was concentrated, and separated and purified by a C-18 reverse-phase chromatographic column. Separation conditions: AQC-18 preparative chromatographic column (Agela40g), 0-50% acetonitrile / TEAA buffer gradient elution, flow rate 20ml / min. The obtained fractions containing the target product were concentrated and kept refrigerated for future use. MS (ESI): Calcd for C 23 h 20 o 5 P(M-H), 407.1. Foun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com