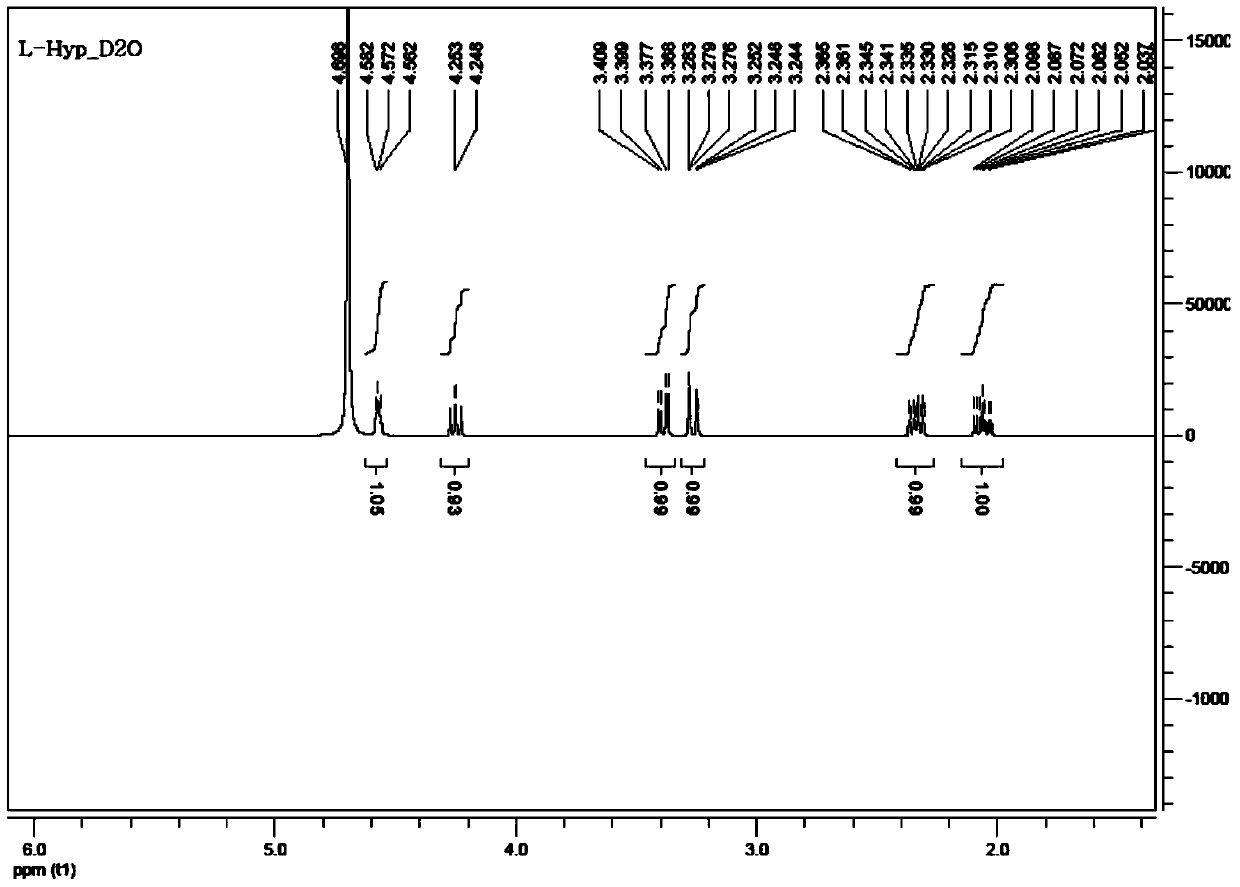
A kind of preparation method of l-hydroxyproline
A technology for hydroxyproline and hydroxyl oxidation, which is applied in the field of preparation of L-hydroxyproline, can solve the problems of drug safety risks, endangering human health, death, etc., and achieve uniform and stable reactions, convenient operation, and expanded applications range effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation method of L-hydroxyproline is as follows:
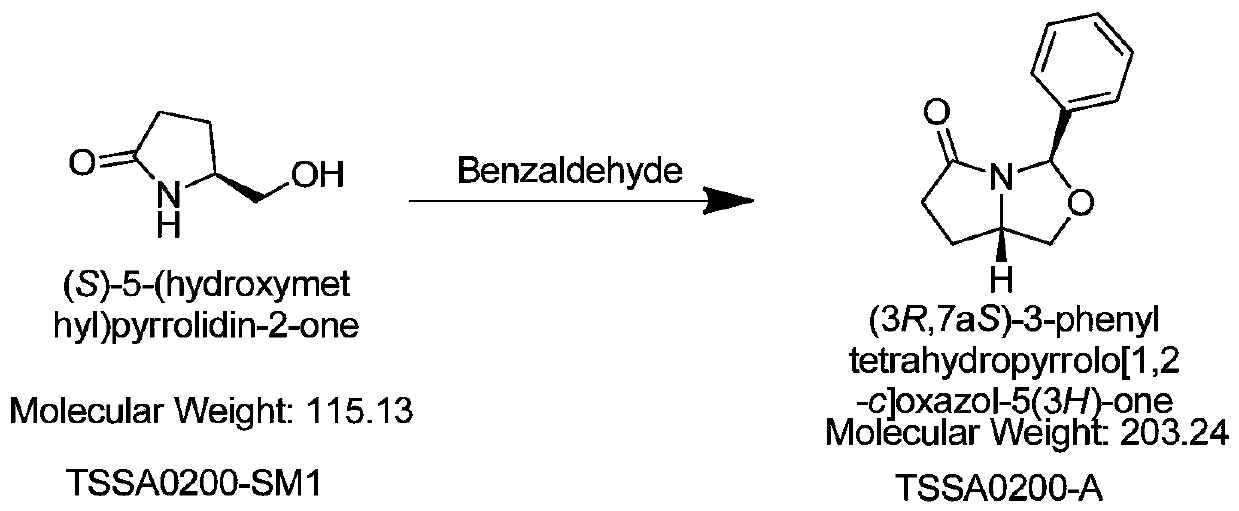
[0043] (1) Add 100g of pyroglutaminol, 16.5g of p-toluenesulfonic acid monohydrate, 130g of benzaldehyde, and 350g of toluene into a 2L reaction flask in sequence, heat at 70-120°C and reflux for 4-6h, evaporate the solvent by distillation under reduced pressure Toluene was used to obtain an oil, which was purified by column chromatography to obtain 40.5g TSSA0200-A, HPLC: 95%, yield: 23%;
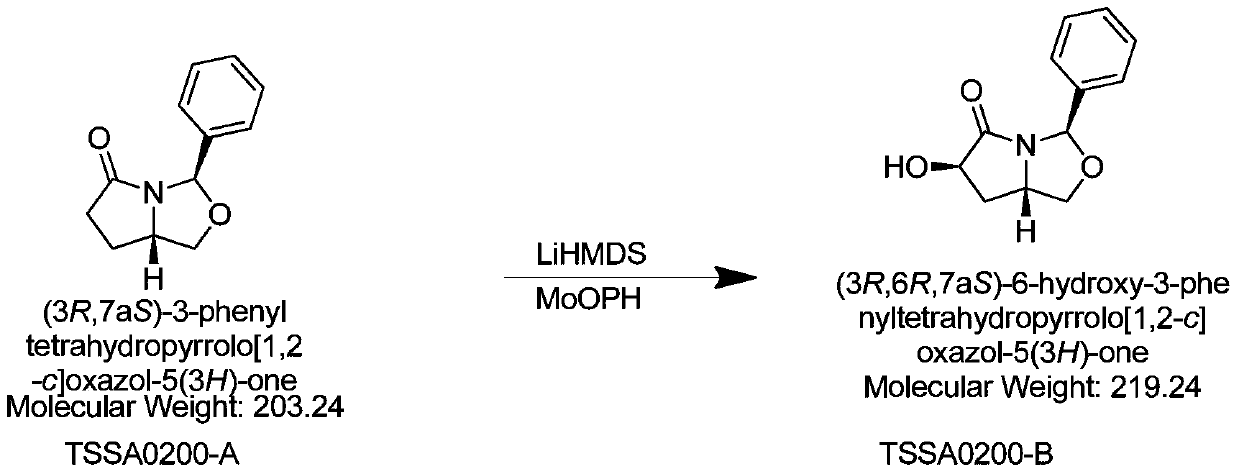
[0044] (2) Take 30g of TSSA0200-A, add 900mL of tetrahydrofuran, add 90g of MoOPH into a 2L reaction flask, lower the temperature to -40~-50℃ under the protection of nitrogen, add LiHMDS 240g dropwise, react for 6-10h, extract the reaction, and the column layer Analysis obtained 9.7gTSSA0200-B, HPLC: 94.2%, yield: 30%;
[0045] (3) Add 5g of TSSA0200-B into a 100mL three-necked flask, add 50mL of tetrahydrofuran under nitrogen protection, cool down to -15~-20°C, add 0.8g of lithium tetrahydrogen, heat up to room temperatur...
Embodiment 2
[0049] The preparation method of L-hydroxyproline is as follows:
[0050] (1) Add 100 g of pyroglutaminol, 20 g of boron trifluoride diethyl ether, 15 g of benzaldehyde, and 450 g of toluene into a 2L reaction flask in sequence, heat at 120°C and reflux for 4-6 hours, distill and concentrate under reduced pressure to evaporate the solvent toluene to obtain an oily substance, Purified by column chromatography to obtain 37.6 g of TSSA0200-A, HPLC: 95%; yield: 21.3%.
[0051] (2) Take 30g of TSSA0200-A, add 900mL of tetrahydrofuran, add 120g of MoOPH into a 2L reaction flask, lower the temperature to -50°C under nitrogen protection, add 300g of potassium hydride dropwise, react for 6-10h, extract the reaction, and perform column chromatography 9.7 g of TSSA0200-B were obtained, HPLC: 94.2%; yield: 30%.
[0052] (3) Add 5 g of TSSA0200-B into a 100 mL three-neck flask, add 50 mL of tetrahydrofuran under nitrogen protection, cool down to -15°C, add 1.2 g of lithium tetrahydrogen a...
Embodiment 3
[0056] The preparation method of L-hydroxyproline is as follows:
[0057] (1) Add 100g of pyroglutaminol, 18g of p-toluenesulfonic acid monohydrate, 80g of benzaldehyde, and 400g of toluene into a 2L reaction flask in turn, heat at 70°C and reflux for 4-6h, distill and concentrate under reduced pressure to evaporate the solvent toluene to obtain an oil The product was purified by column chromatography to obtain 39.5g TSSA0200-A, HPLC: 95%; 22.4%.
[0058] (2) Take 30g of TSSA0200-A, add 900mL of tetrahydrofuran, add 100g of MoOPH into a 2L reaction flask, lower the temperature to -40°C under the protection of nitrogen, add LiHMDS260g dropwise, react for 6-10h, extract the reaction, and obtain 9.7g by column chromatography TSSA0200-B, HPLC: 94.2%; 30%.
[0059] (3) Add 5 g of TSSA0200-B into a 100 mL three-necked flask, add 50 mL of tetrahydrofuran under nitrogen protection, cool down to -15°C, add 1 g of sodium borohydride, heat up to room temperature and react for 6-8 hours,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com