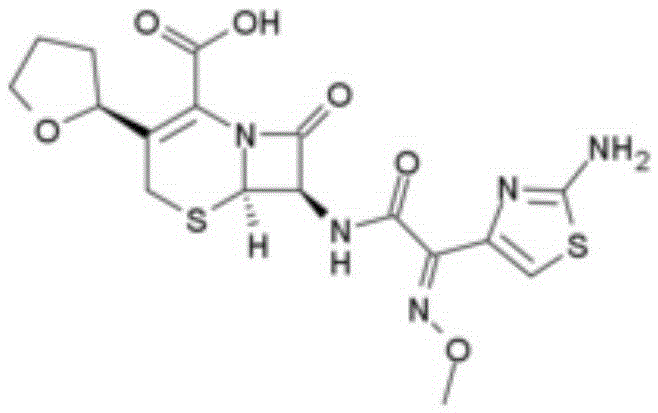
Convenia synthetic method and Convenia sodium salt synthetic method
A synthesis method and technology of cefvecin, applied in the field of pharmaceutical compounds, can solve problems such as unfavorable operation, cost saving, harsh reaction conditions, and difficult operation, and achieve the effects of low equipment requirements, mild reaction conditions, and improved process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] A kind of synthetic method of cefotaxime of the present invention comprises the following steps:
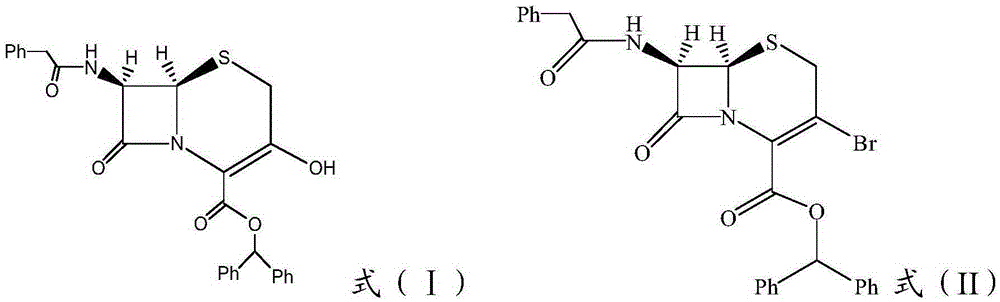
[0033] (1) Bromination: dissolve ceftizoxime sodium (GHYA) intermediate formula (I) in an organic solvent, then cool down to 3°C, slowly add brominating agent dropwise, then add alkali, and react at 0-5°C for 2 Hours, after the reaction is complete, adjust the pH to 2, add water, extract, the water phase is sequentially extracted with petroleum ether and an organic solvent, adjust the pH of the water phase to 9, and after the organic solvent extraction, concentrate and dry to obtain the brominated product formula (II);
[0034]
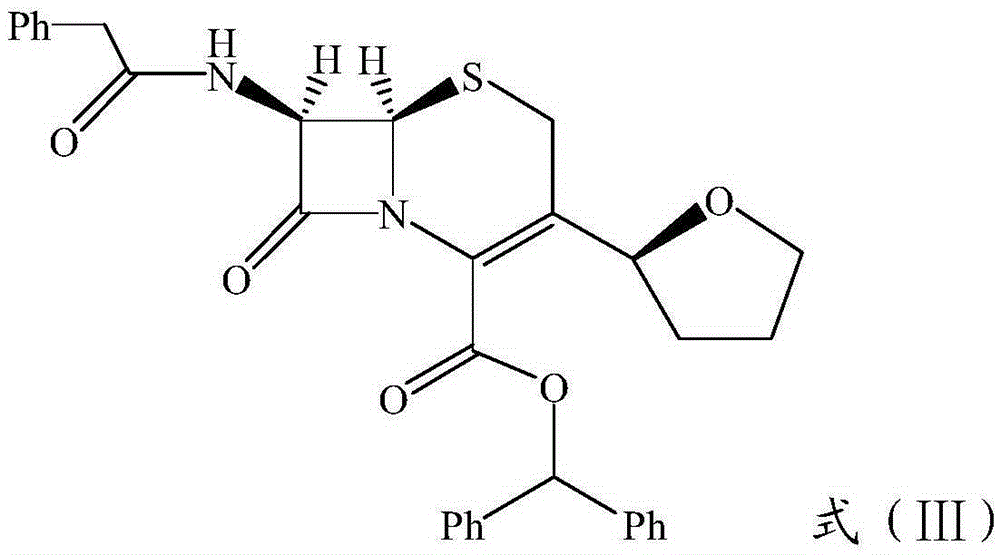
[0035] (2) Electrophilic addition: Dissolve dihydrofuran in toluene, add benzenesulfinic acid and HBF at room temperature 4 organic solvent, stirred for reaction, extracted with ethyl acetate, concentrated, dried, and HPLC chiral separation to obtain white solid product benzenesulfinic acid-2S-tetrahydrofuryl ester;
[0036] (3) Nucleophilic s...
Embodiment 1
[0055] (6R,7R)-3-Bromo-8-oxo-7-[(phenylacetyl)amino]-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2- Synthesis of Diphenylmethyl Carboxylate:
[0056] In the low-temperature reaction tank, add 20gGHYA and 320ml dichloromethane into a 500ml three-necked flask, then cool to 3°C, stir, and slowly add 10.8ml of phosphorus tribromide in dichloromethane solution dropwise for 10 minutes, then slowly Slowly add 5.2ml of triethylamine dropwise, the pH of the reaction solution is slightly alkaline, stir at 3°C for 2 hours, and monitor the end point of the reaction by thin-layer chromatography. After the reaction is complete, add 150ml of distilled water, adjust the pH to 2 with 3N HCL, separate the liquids, extract the organic phase with an acidic water phase, combine the water phases, and extract the water phase with petroleum ether and dichloromethane twice, keep the water phase, and then Add 150ml of dichloromethane, adjust the pH to 9 with 5N NaOH, separate the layers, extract the aqueous...
Embodiment 2
[0059] Synthesis of benzenesulfinic acid-2S-furyl ester:
[0060] Add 5g of benzenesulfinic acid, 2.8g of dihydrofuran, and 60ml of toluene into a 250ml three-necked flask, cool down to 10°C, and add 60ml containing 0.5g of HBF 4 dichloromethane solution, stirred and reacted at 25°C for 2 hours, and the end point of the reaction was monitored by thin-layer chromatography. After the reaction was completed, 50ml of water was added, and the aqueous phase was separated. The organic phase was extracted twice with the aqueous phase, and the organic phase was dried over anhydrous sodium sulfate. Concentrated under reduced pressure, dried in vacuo, and chiral separation by HPLC (n-hexane:isopropanol) gave 3.34g of benzenesulfinic acid-2R-furyl ester, yield 44.7%, melting point 61-63°C, MS-ESI (M+1) :213.26; NMR results are as follows:
[0061] 1 HNMR (DMSO) δ: 7.52 (d, 2H), 7.41 (d, 2H), 4.67 (s, 2H);
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com