Method for preparing midbody of heart failure medicine
A compound and reaction technology, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of harsh reaction conditions and high risk factors, and achieve the effects of simple operation, shortened reaction steps, and low cost of synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
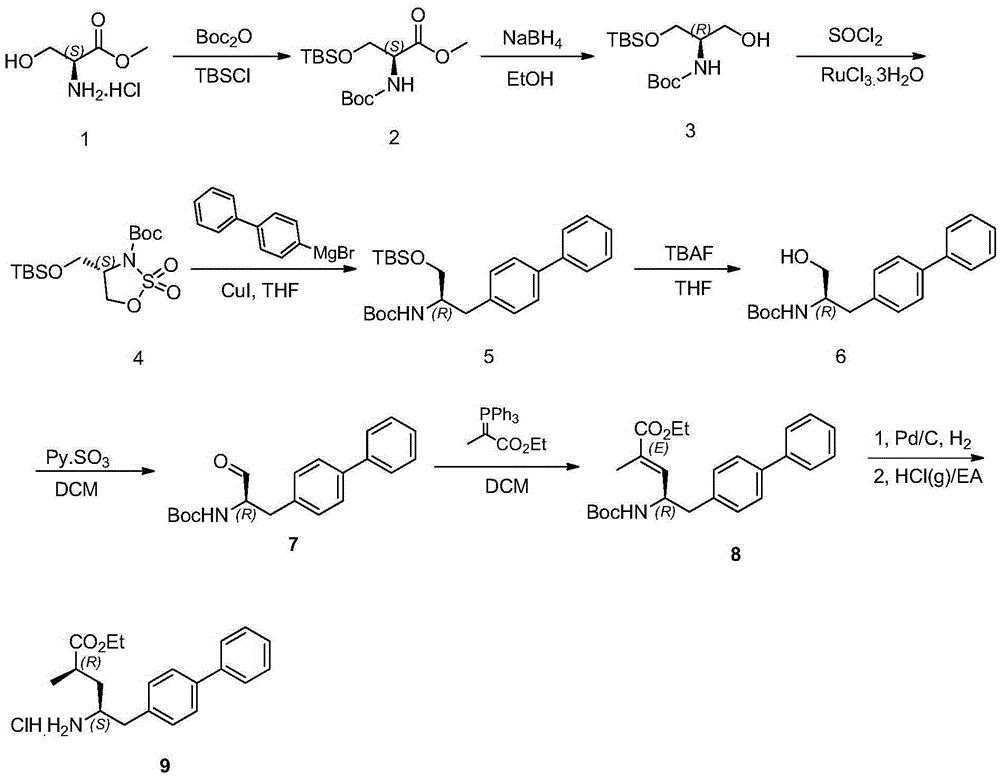
[0072] Disclosed in this embodiment is a method for preparing a compound of formula A, comprising the following steps:
[0073] a) Add compound 1L-serine methyl ester hydrochloride (155.58g, 1mol, 1eq) and triethylamine (303g, 3mol, 3eq) into dichloromethane (1.55L), and control the temperature at 10°C to 25°C After adding BOC anhydride (222.36g, 1.02mol, 1.02eq) dropwise, stir at room temperature until the reaction is complete, filter with suction, add water solution, wash the organic phase with hydrochloric acid (1mol / L) to PH=7, dry, remove the desiccant, Cool to 0°C, add tert-butyldimethylsilyl chloride (153.73g, 1.02mol, 1.02eq), and finally dissolve imidazole (201g, 3mol, 3eq) in dichloromethane (200ml) and add dropwise to the reaction solution, Then naturally warming up to room temperature to react, after the reaction is complete, quench the reaction with 2mol / L hydrochloric acid, adjust the pH to 5, separate the liquids, extract the aqueous phase with dichloromethane, ...
Embodiment 2
[0077] A method for preparing a compound of formula B is disclosed in this embodiment, specifically:
[0078] Add biphenylmagnesium bromide Grignard reagent (1.1L, 1.1mol, 1.1eq) into anhydrous tetrahydrofuran (550mL), control the temperature at 0 degrees, add cuprous iodide (3.8g, 0.02mol, 0.02eq) , stirred at this temperature for one hour, the compound tert-butyl (S)-4-(((tert-butyldimethylsilyl)oxy)methyl)-1,2,3-oxothiazole-3-carboxy Acid 2,2-dioxide (367g, 1mol, 1eq) was dissolved in tetrahydrofuran (1050mL), added dropwise to the reaction solution at 0°C, stirred and reacted, and after the reaction was complete, 10% citric acid aqueous solution (1000mL ) to quench the reaction, stir for half an hour, separate the liquids, and concentrate the organic phase under reduced pressure to obtain a white solid, which is recrystallized with ethyl acetate and petroleum ether to obtain tert-butyl (R)-(1-([1,1'-biphenyl ]-4-yl)-3-((tert-butyldimethylsilyl)oxy)propan-2-yl)carbamate. ...
Embodiment 3
[0080] This example is a compound A or compound B used to prepare tert-butyl (R)-(1-([1,1'-biphenyl]-4-yl)-3-hydroxypropan-2-yl)amino The method for formic acid also includes the steps:
[0081]The compound tert-butyl (R)-(1-([1,1'-biphenyl]-4-yl)-3-((tert-butyldimethylsilyl)oxy)propan-2-yl) Carbamic acid (442g, 1mol, 1eq) was dissolved in tetrahydrofuran (2200mL), a THF solution of tetrabutylammonium fluoride (2L, 2mol, 2eq) was added, and the reaction was completed at room temperature. The reaction solution was diluted with ethyl acetate, and water was added. The reaction was quenched, separated, the aqueous phase was extracted with ethyl acetate, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed to obtain an off-white solid, which was recrystallized with ethyl acetate and n-heptane to obtain The white solid is tert-butyl(R)-(1-([1,1'-biphenyl]-4-yl)-3-hydroxypropan-2-yl)carbamate. In this step, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com