Chenxiang Lubailu medicine preparation
A pharmaceutical preparation, white dew technology, applied in the field of Chen Xianglu Bailu pharmaceutical preparations and its preparation, can solve the problems of low bioavailability, incomplete absorption, slow curative effect, etc., achieve better qi and pain relief, good bioavailability, and release high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
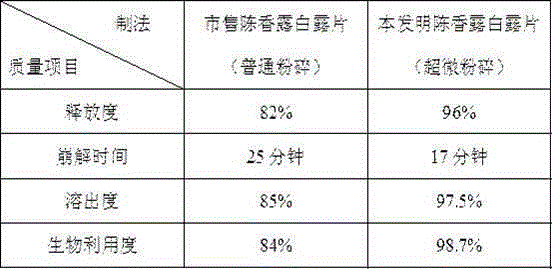
[0038] Embodiment 1 (Chenxianglu Bailu tablets of the present invention)
[0039] prescription: 100g of licorice, 100g of tangerine peel, 38.2g of Chuanxiang, 8.3g of rhubarb, 9.2g of calamus, 110g of bismuth subnitrate, 33.7g of magnesium carbonate, 33.7g of magnesium oxide, and 67g of sodium bicarbonate.
[0040] Preparation:
[0041] 1) Firstly crush licorice, tangerine peel, chrysanthemum, rhubarb, and calamus into fine powder with a superfine pulverizer, then weigh the five-flavored Chinese medicine powder according to the prescription, and mix through a 200-300 mesh sieve;
[0042] 2) Take bismuth subnitrate to make colloidal bismuth through colloid mill, add warm distilled water and stir for 5-10 minutes to make bismuth subnitrate liquid;
[0043] 3) Weigh magnesium carbonate, magnesium oxide, sodium bicarbonate three flavor chemicals and starch according to the prescription and mix and stir;
[0044] 4) Mix 1) and 3), pass the material through a 40-120 mesh siev...
Embodiment 2
[0045] Example 2 (Commercially available Chenxianglu Bailu Tablets)
[0046] Prescription: licorice 100g, bismuth subnitrate 110g, tangerine peel 100g, magnesium carbonate 33.7g, Chuanxiang 38.2g, magnesium oxide 33.7g, rhubarb 8.3g, sodium bicarbonate 67g, Shichangpu 9.2g.
[0047] Preparation:
[0048] 1) Grind licorice, tangerine peel, chrysanthemum, rhubarb, and calamus into 80-mesh fine powder, then weigh five-flavored Chinese medicine powder according to the prescription, and pass through a 18-mesh sieve; weigh bismuth subnitrate, magnesium carbonate, and magnesium oxide according to the prescription , sodium bicarbonate four chemicals and starch are mixed and stirred, and passed through a 40-mesh sieve;
[0049] 2) Mix the materials in step 1) in the mixer for 5 minutes, add warm distilled water and stir for 5-10 minutes to make a suitable soft material. After sizing with a 14-mesh nylon mesh, the coarse particles are crushed with a dust mill (coarse mesh) Mix with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com