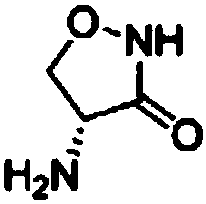
A kind of preparation method of d-cycloserine
A technology of cycloserine and serine, applied in the directions of organic chemistry, antibacterial drugs, etc., can solve the problems of low reaction yield, unstable product quality, complicated post-processing operations, etc., and achieves easy availability of raw materials, improved total reaction yield, The effect of stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
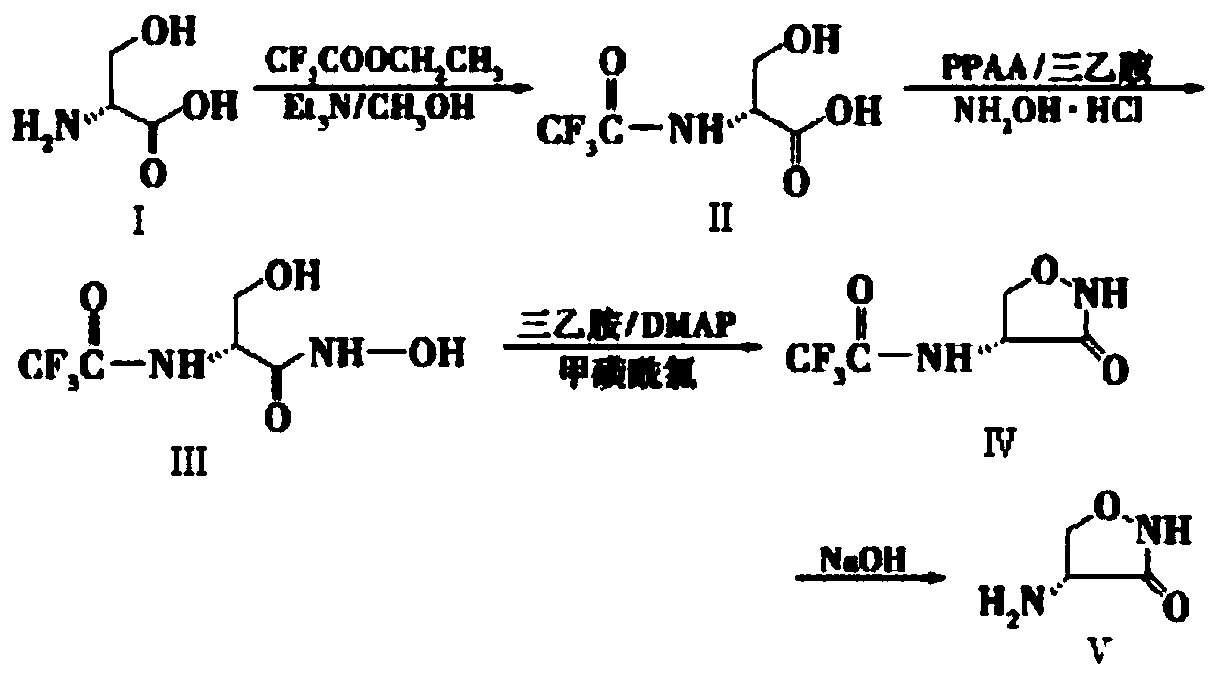
[0029] (1) Preparation of N-trifluoroacetyl-D-serine
[0030] Add 21.0g of D-serine to a mixed solvent of 140ml of methanol and 40ml of triethylamine, add 18g of ethyl trifluoroacetate under nitrogen protection, react at 20-25°C for 14h, cool to 5°C, and filter to obtain 39.0g of crude compound II , yield 97.1%.
[0031] (2) Preparation of (1-trifluoroacetylamino-2-hydroxyl) ethyl hydroxamic acid
[0032] Mix 61.7g PPAA and 57.6g triethylamine evenly and add to 39.0g compound II obtained in the above step (1), then add 80ml ethyl acetate and 400ml acetonitrile, stir at room temperature for 0.5h, then add 26.4g hydroxylamine hydrochloride, After stirring and reacting for 10 h, it was washed with 600 ml of saturated brine, the organic phase was dried over anhydrous sodium sulfate, and the solvent was recovered by distillation, and the concentrated solution was cooled at room temperature for use.
[0033] (3) Preparation of D-4-trifluoroacetylamino-3-oxazolidinone
[0034] Aft...
Embodiment 2
[0038]Step (2) Preparation of (1-trifluoroacetylamino-2-hydroxyl) ethyl hydroxamic acid: 57.4g PPAA and 38.5g triethylamine are mixed uniformly and then added to compound II obtained in the above step (1), and then added 80ml of ethyl acetate and 400ml of acetonitrile, stirred at room temperature for 0.5h, then added 26.4g of hydroxylamine hydrochloride, stirred at room temperature for 10h, washed with 600ml of saturated saline, dried the organic phase with anhydrous sodium sulfate, recovered the solvent by distillation, and cooled the concentrate at room temperature stand-by. Other steps adopt the same operating process as in Example 1 to obtain 10.1 g of D-cycloserine with a total yield of 52.1% and a content of over 98%.
Embodiment 3
[0040] Step (3) Preparation of D-4-trifluoroacetamido-3-oxazolidinone: Mix 57.6g of anhydrous triethylamine, 7.0g of DMAP and 400ml of anhydrous toluene and add to step (2) to prepare In the concentrated solution of Methanesulfonyl chloride, slowly add the anhydrous toluene solution of methanesulfonyl chloride (17.4g methanesulfonyl chloride is dissolved in 120ml of anhydrous toluene) to the reaction system, react at room temperature for 4 hours after the drop, and recover the solvent toluene by distillation under reduced pressure to obtain Concentrate containing compound IV. Other steps adopt the same operating process as in Example 1 to obtain 7.8 g of D-cycloserine with a total yield of 40.3% and a content of over 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com