Transdermal formulations
一种透皮、溶剂的技术,应用在无水透皮制剂领域,能够解决炎症、皮肤刺激等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
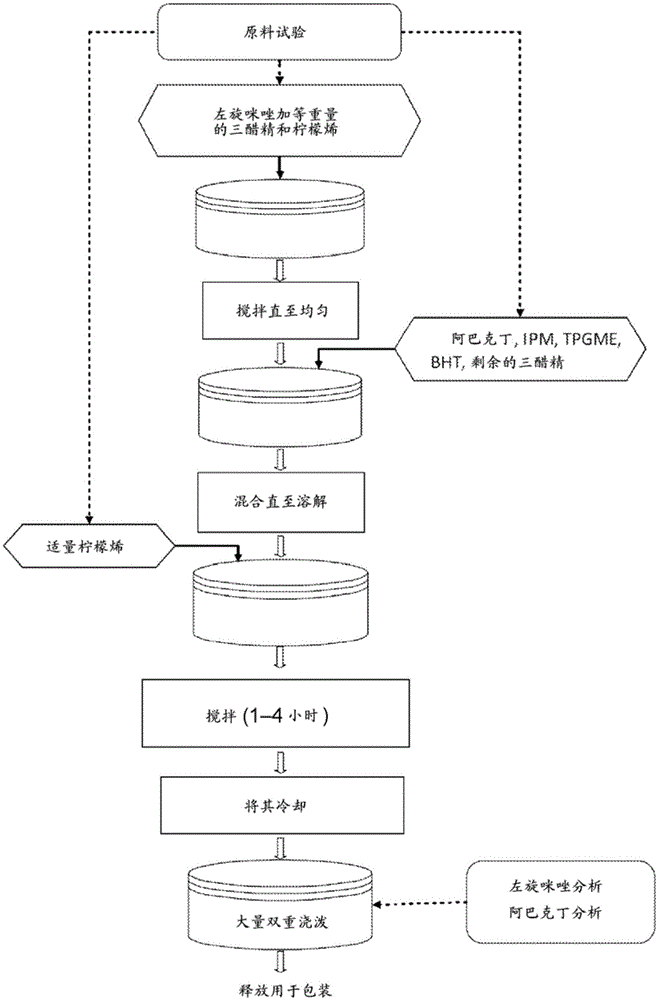
Image
Examples
Embodiment 1
[0404] Example 1 - Stability Study
[0405] 1. Solvent stability
[0406] The purpose of these studies is to identify solvents and co-solvents to optimize active substance solubility and product physical / chemical stability.
[0407] In a certain range of solvents, the solubility and recovery of levamisole base and / or abactin after one month were determined, as shown in Table 1.
[0408] Table 1. Solubility data for solvents used alone to examine the stability of two actives, levamisole base and abactin
[0409]
[0410]
[0411]
[0412] 2. Abaktin stability
[0413] The purpose of this study was to examine the stability of abactin in a solvent-neutralized solvent system containing only glycerol formal (GF) and a solvent system comprising a complexing agent (eg PVP), an acidulant (eg malic acid) and Chelating agents such as EDTA are combined with glycerol formal.
[0414] Table 2. Stability data for levamisole base and abactin in solvent systems containing glycero...
Embodiment 2
[0456] Example 2 - In vitro studies
[0457] 5. Permeability studies
[0458] This example describes the use of a platform composition to deliver a range of different actives across the skin of a range of different animals (bovine, equine, rabbit).
[0459] The active substances investigated in this example are
[0460] ■Levamisole base (insect repellent)
[0461] ■ Macrolides (abactin and moxidectin) (insect repellants),
[0462] Hydrocortisone (steroidal anti-inflammatory agent),
[0463] ■ metoclopramide (antiemetic),
[0464] cetirizine (an antihistamine), and
[0465] ■ Diphenhydramine (antihistamine).
[0466] The properties of each active tested are shown in Table 7 below.
[0467] Table 7. Characteristics of the tested APIs
[0468]
[0469]
[0470] Formulations of the platform compositions are given in Table 8.
[0471] Table 8. Formulations for Abaktin / Levamisole Base Formulations
[0472]
[0473] Table 9. Formulations for Moxidectin / Levamisole B...
Embodiment 3
[0570] Example 3 - Clinical Study
[0571] Two clinical efficacy studies were conducted. The study design for each study is summarized below.
[0572] ■Study 1 - Winter Fur
[0573] ○ Control
[0574] ○ When there is no rain, test the composition
[0575] ○ Rain after 2 hours of application, test composition
[0576] ■Study 2 - Summer Fur
[0577] ○ Control
[0578] ○Test composition
[0579] ○Comparative product (Eclipse-combination dual pour-on with abactin and levamisole)
[0580] ○Single active substance comparison product
[0581] The purpose of Study 1 was to evaluate the efficacy of the test composition against gastrointestinal parasites in cattle with winter coats and to determine the effect of rain on the efficacy of the composition after application of the composition on the skin of cattle.
[0582] The purpose of Study 2 was to evaluate the efficacy of the test composition against gastrointestinal parasites in cattle with summer coats and to compare with th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com