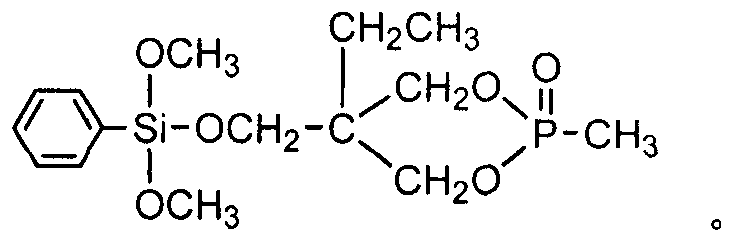
Preparing method for phenyl dimethoxy (phosphor heterocycle methoxyl) silicane compound
A technology of phenyldimethoxy and flame retardant phenyldimethoxy, which is applied in the field of preparation of flame retardant phenyldimethoxysilane compounds, can solve the threat to life safety, poor electrical performance, use of Restriction and other problems, to achieve high decomposition temperature, promote flame retardant effect, prevent melting and dripping effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
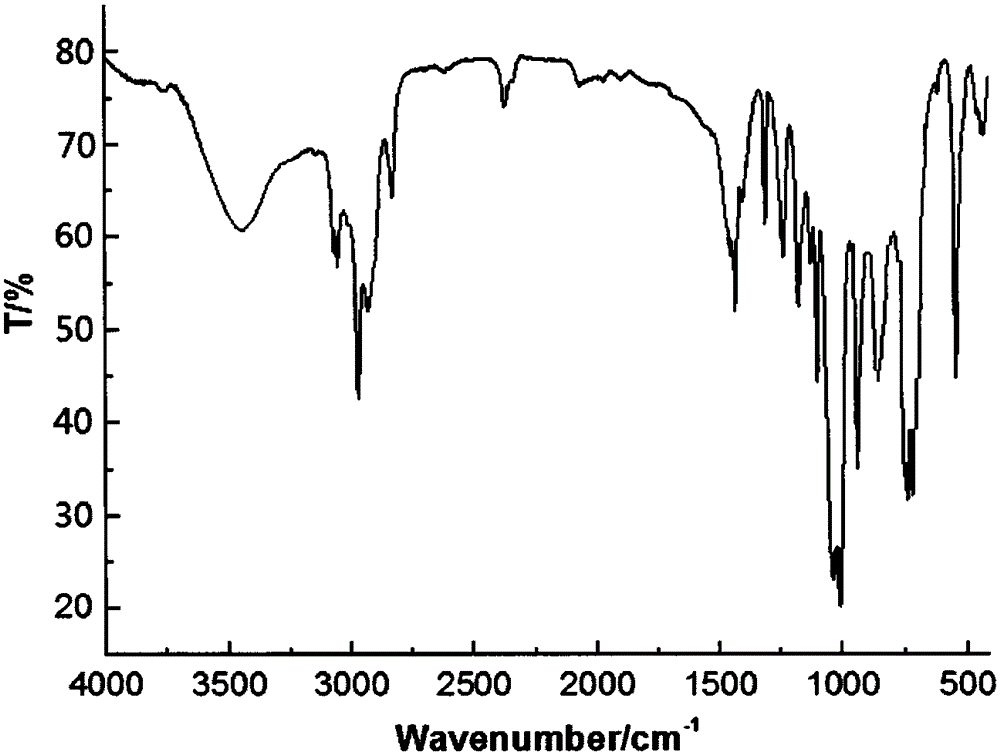
[0024] Example 1 In a 200ml four-neck flask equipped with a stirrer, a thermometer, and a high-efficiency fractionation device, replace the air in the bottle with nitrogen, and add 19.40g (0.10mol) of 4-hydroxymethyl-4-ethyl-cycloformazol Base phosphonate, 19.83g (0.10mol) phenyltrimethoxysilane, 40ml diethylene glycol dimethyl ether, heat up to 140°C, control the fractionation column top temperature not higher than 65°C, fractionate the generated methanol, and react 5h, after no methanol is produced, change it to a vacuum distillation device, diethylene glycol dimethyl ether (recovered and used) is removed by vacuum distillation, then add 35ml of toluene, stir, transfer to a separatory funnel to stand for stratification, The lower layer material liquid was separated, and a small amount of toluene and low boiling point substances were removed by distillation under reduced pressure to obtain light yellow liquid phenyldimethoxy (phosphacyclomethoxy) silane with a product yield of...
Embodiment 2
[0025] Example 2 In a 200ml four-neck flask equipped with a stirrer, a thermometer, and a high-efficiency fractionation device, replace the air in the bottle with nitrogen, and add 19.40g (0.10mol) of 4-hydroxymethyl-4-ethyl-cycloformazol Base phosphonate, 25.78g (0.13mol) phenyltrimethoxysilane, 50ml ethylene glycol diethyl ether, raise the temperature to 120°C, control the temperature at the top of the fractionation column to not be higher than 65°C, fractionate the generated methanol, and react for 7h. After no methanol is produced, change it to a vacuum distillation device, and remove ethylene glycol diethyl ether (recovery and use) by vacuum distillation, then add 70ml of toluene, stir, transfer to a separatory funnel to stand for stratification, and separate the lower layer of material Liquid, a small amount of toluene and low boiling point substances were distilled off under reduced pressure to obtain light yellow liquid phenyl dimethoxy (phosphacyclomethoxy) silane, and...
Embodiment 3
[0026] Example 3 In a 200ml four-necked flask equipped with a stirrer, a thermometer, and a high-efficiency fractionation device, replace the air in the bottle with nitrogen, and add 19.40g (0.10mol) of 4-hydroxymethyl-4-ethyl-cycloformazol Base phosphonate, 27.76g (0.14mol) phenyltrimethoxysilane, 80ml DMF, heat up to 130°C, control the temperature at the top of the fractionation column to not be higher than 65°C, fractionate the generated methanol, fractionate for 6 hours, and wait until no methanol is produced Afterwards, change it into a vacuum distillation device, and remove DMF (recovery and use) by distillation under reduced pressure, then add 50ml of toluene, stir, transfer to a separatory funnel and leave it to stand for layering, separate the lower floor material liquid, and remove a small amount of toluene by distillation under reduced pressure. And low boiling point, to obtain light yellow liquid phenyl dimethoxy (phosphacyclomethoxy) silane, product yield 96.8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com