A kind of preparation method of 2,2-difluoropiperic acid methyl ester
A technology of methyl difluropiperate and difluoropiperic acid is applied in the field of preparation of methyl 2,2-difluoropiperonate, and can solve the problems of easily polluted environment, difficult control of reaction process, complicated purification process, etc. The effect of easy reaction process, high product yield and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
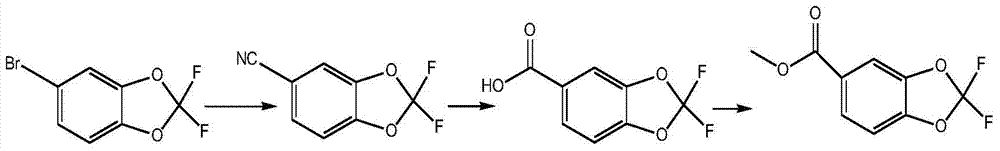
[0027] Put 660g of DMF, 110g of lithium bromide, and 110g of cuprous cyanide into the dry reaction tank and stir evenly to dissolve the lithium bromide and cuprous cyanide, then add 237g of 5-bromo-2,2-difluoropipercycline, and keep the reaction at 130°C After 5 hours of reaction, cool to 20°C, add 2.7kg of water, and steam distill to obtain a solid product, which is 162g after drying, and the content of 162g of 5-cyano-2,2-difluoropiperone is 99.2%. The yield 88.5%.
[0028] Put 820g of water and 141g of sodium hydroxide into the reaction tank and stir evenly to dissolve the sodium hydroxide, add 162g of 5-cyano-2,2-difluoropipercycline, reflux for 5h, cool to 20°C after the reaction, When it is below 30°C, adjust the pH of the system to 2 with hydrochloric acid with a mass concentration of 30%. The product obtained by suction filtration was 172g after drying, in which the content of 2,2-difluoropiperic acid was 99.6%, and the yield was 96.2%.
[0029] Put 860g of methanol ...
Embodiment 2
[0031] Put 940g of sulfolane, 130g of lithium bromide, and 130g of cuprous cyanide into the dry reaction tank and stir evenly to dissolve the lithium bromide and cuprous cyanide, then add 237g of 5-bromo-2,2-difluoropiperone ring, and keep the reaction at 135°C After 8 hours of reaction, cool to 20°C, add 2.9kg of water, and steam distill to obtain a solid product, which is 167g after drying, and the content of 167g of 5-cyano-2,2-difluoropiperone ring is 99.3%. The yield 91.2%.
[0032] Put 1280g of water and 216g of sodium hydroxide into the reaction tank and stir evenly to dissolve the sodium hydroxide, add 167g of 5-cyano-2,2-difluoropipercycline, reflux for 4 hours, cool to 20°C after the reaction, When it is below 30°C, adjust the pH of the system to 3 with hydrochloric acid with a mass concentration of 30%. The product obtained by suction filtration was 170g after drying, and the content of 2,2-difluoropiperic acid was 99.7%, and the yield was 92.2%.
[0033] Put 1100...
Embodiment 3
[0035] Add 460g of dimethyl sulfoxide, 115g of lithium bromide, and 115g of cuprous cyanide to the dry reaction tank and stir evenly to dissolve lithium bromide and cuprous cyanide, then add 237g of 5-bromo-2,2-difluoropipercycline, Reaction at 125°C for 8 hours. After the reaction, cool to 20°C, add 3.5kg of water, and steam distill to obtain a solid product, which is 164g after drying, and the content of 164g of 5-cyano-2,2-difluoropipercycline is 99.4 %, yield 89.6%.
[0036] Put 1020g of water and 144g of sodium hydroxide into the reaction tank and stir evenly to dissolve the sodium hydroxide, add 164g of 5-cyano-2,2-difluoropipercycline, reflux for 7 hours, cool to 20°C after the reaction, When it is below 30°C, adjust the pH of the system to 2 with hydrochloric acid with a mass concentration of 30%. The product obtained by suction filtration was 171g after drying, the content of 2,2-difluoropiperic acid was 99.4%, and the yield was 94.4%.
[0037] Put 1197g of methanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com