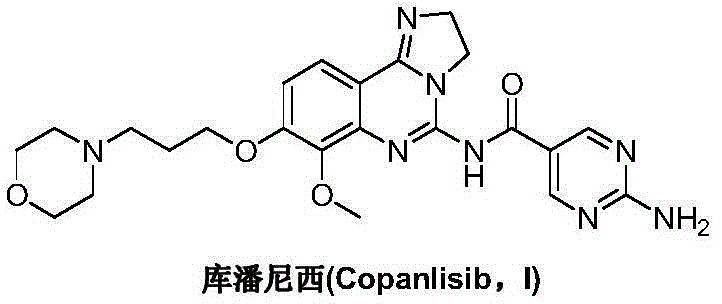
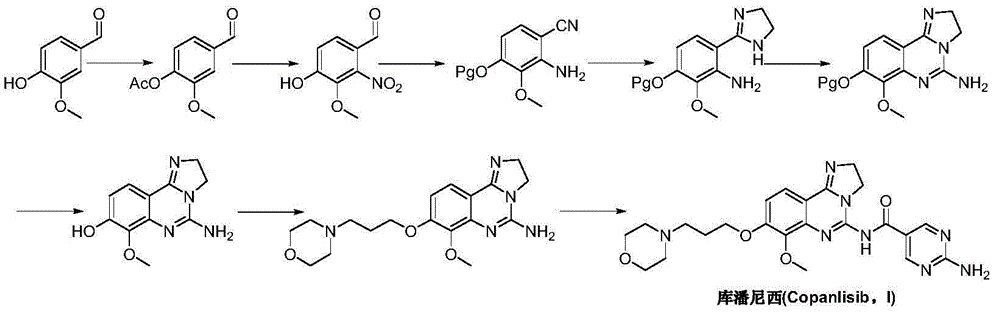
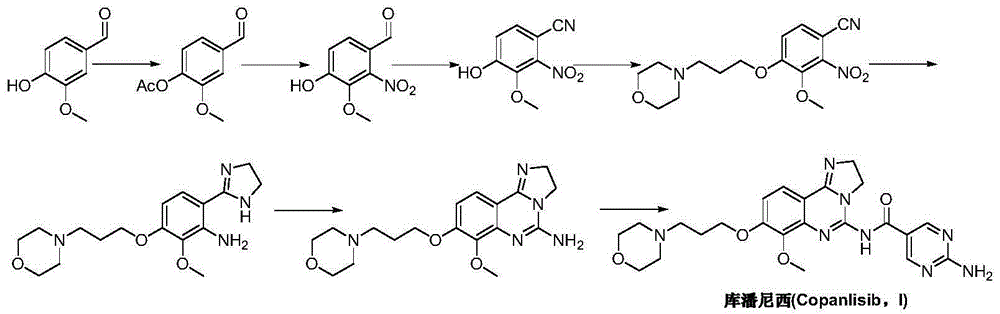
Preparation method for copanlisib
A technology of morpholine and oxypropoxy, which is applied in the field of preparation of the drug Copanicil, can solve problems such as side reactions, product quality and adverse effects of the purification process, and achieve the effects of easy availability of raw materials, promotion of development, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]In a nitrogen atmosphere, add 2-amino-3-methoxy-4-(3-morpholin-4-ylpropoxy)benzonitrile (II) (5.82g, 20mmol) and dioxane in a dry reaction flask 50 mL was collected, and benzoyl isocyanate (3.23 g, 22 mmol) was added at room temperature and stirred at room temperature for 20 hours. TLC detected that the reaction was complete. Stand still, filter, the filter cake is washed with a mixed solvent of n-hexane and ethyl acetate (4:1), and dried in vacuo to obtain off-white solid 4-amino-7-(3-morpholin-4-ylpropoxy)-8 -Methoxyquinazolin-2(1H)-one (III) 4.55 g, yield 68.1%; mass spectrum (EI): m / z 335 (M+H). Embodiment two:
Embodiment 2
[0045] In a nitrogen atmosphere, add 2-amino-3-methoxy-4-(3-morpholin-4-ylpropoxy)benzonitrile (II) (5.82 g, 20 mmol) and 50 mL of tetrahydrofuran into a dry reaction flask, A solution of chlorosulfonic acid isocyanate (3.25 g, 23 mmol) in tetrahydrofuran (15 mL) was added dropwise at 0-5°C. After dropping, warm up to room temperature, stir and react for 6-8 hours, and TLC detects that the reaction is complete. The pH was adjusted to neutral with 5% sodium hydroxide, and extracted three times with dichloromethane. The organic phases were combined and washed with water and saturated brine. Concentrate under reduced pressure, the residue is washed with a mixed solvent of n-hexane and ethyl acetate (4:1), and dried in vacuo to give an off-white solid 4-amino-7-(3-morpholin-4-ylpropoxy)-8- Methoxyquinazolin-2(1H)-one (III) 5.10 g, yield 76.3%; mass spectrum (EI): m / z 335 (M+H).
Embodiment 3
[0047] In a nitrogen atmosphere, add 4-amino-7-(3-morpholin-4-ylpropoxy)-8-methoxyquinazolin-2(1H)-one (III) (3.3g , 10mmol), 2-chloroethanol (0.97g, 12mmol), potassium tert-butoxide (1.68g, 15mmol) and N,N-dimethylformamide 25mL, heated to 80-90°C, stirred for 4-6 hours . Cool down to room temperature, pour the reaction solution into a 5% sodium hydroxide solution by weight, heat to 60°C, keep warm for 2 hours, cool to room temperature, solids precipitate out, filter, wash with water, wash with n-hexane, and dry in vacuum Yellow solid 7-methoxy-8-(3-morpholin-4-ylpropoxy)-2,3-dihydroimidazo[1,2-c]quinazolin-5(6H)-one ( IV) 2.12 g, yield 58.9%; mass spectrum (EI): m / z 361 (M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com