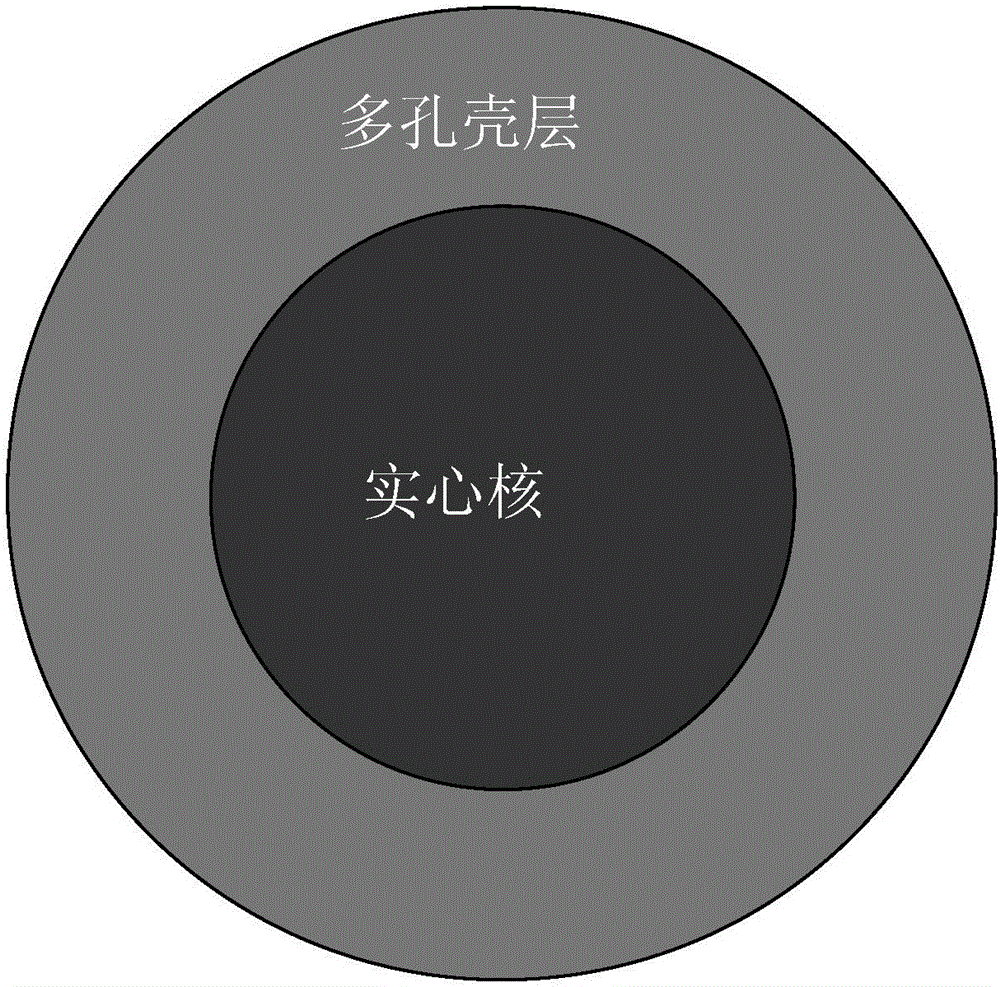
Core-shell type porous zirconium dioxide material and preparation method thereof
A technology of zirconia and nano-zirconia, which is applied in zirconia, chemical instruments and methods, and other chemical processes, and can solve the problems of limited pressure range of full-pore packing, mass transfer process and long separation time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Dissolve 200 g of zirconium n-propoxide in a mixed solution of 2500 mL of ethanol and 1000 mL of deionized water. 20 mL of 30% ammonia solution was added, mechanically stirred at 600 rpm for 30 seconds at 35° C., and then the mixture was slowly magnetically stirred at room temperature for 3 hours to generate nano-sized zirconia particles. Distill to reduce the volume of the mixture to 800mL, and filter to obtain a colorless, clear high-purity zirconia sol, and add a small amount of ammonia to adjust the pH to about 7-8. The content of nano zirconium dioxide particles in the zirconium dioxide sol is 6.5%, and the particle diameter is 25nm.
Embodiment 2
[0042] Dissolve 150 g of zirconium isopropoxide in a mixed solution of 2000 mL of ethanol and 500 mL of deionized water. 5 mL of 30% ammonia solution was added, mechanically stirred at 600 rpm for 30 seconds at 25° C., and then the mixture was slowly magnetically stirred at room temperature for 3 hours to generate nano-sized zirconia particles. Distill to reduce the volume of the mixture to 500mL, and filter to obtain a colorless, clear high-purity zirconia sol, and add a small amount of ammonia to adjust the pH to about 8-9. The content of nano zirconium dioxide particles in the zirconium dioxide sol is 7.1%, and the particle diameter is 15nm.
Embodiment 3
[0044] Add 1240 mL of deionized water, 37 g of urea, 60 g of 37% formaldehyde solution, and the zirconium sol prepared in Example 1 into a 5000 mL beaker in sequence, and stir until the urea dissolves.
[0045] Add 2 mL of nitric acid under rapid stirring and continue rapid stirring for 15 seconds. After standing for 30 minutes, a white precipitate, that is, zirconium dioxide-urea-formaldehyde resin composite microspheres, was precipitated.
[0046] Filtration, washing, drying, and microscopic examination revealed that the particle size of the zirconia composite microspheres was 2.5 μm, and the spherical shape was uniform.
[0047] The above-mentioned zirconia composite microspheres were calcined at a high temperature of 1100° C. for 15 hours. 50 g of zirconia solid spheres were obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com