Cefamandole nafate composition for the treatment of infectious diseases
A technology of cefamandole sodium and infectious diseases, which is applied in the field of medicine, can solve the problems of structural damage, unfavorable stability, and great harm to patients, and achieve the effects of low content of insoluble particles, suitable for clinical application, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
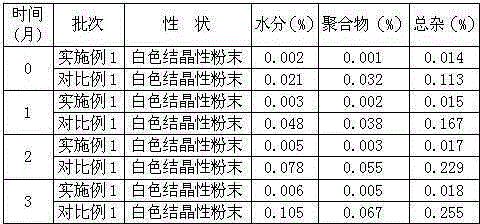
Image
Examples
Embodiment 1
[0026] Example 1: Preparation of Cefamandole Sodium Crystals
[0027] (1) Grinding the crude product of cefamandole sodium, passing through a 60-mesh sieve, then adding it to deionized water whose volume is 6 times the weight of cefamandole sodium, and stirring at 130 rpm for 10 minutes;
[0028] (2) Add methanol whose volume is 4 times the weight of cefamandole sodium under stirring at 90 rpm, and raise the temperature to 30°C at the same time;
[0029] (3) After the solution is added, let it stand for 3 hours, and add dropwise a mixed solution of ethanol and chloroform whose volume is 8 times the weight of cefamandole sodium at 0°C under stirring at 150 rpm, and a mixture of ethanol and chloroform The volume ratio is 3:1, and the uniform dropwise addition is completed within 2 hours;
[0030] (4) After the dropwise addition, cool down to -5°C, continue to stir at a stirring rate of 80 rpm for 2 h, let stand for 1 h to precipitate crystals, filter, wash, and dry in vacuo t...
Embodiment 2
[0032] Example 2: Preparation of cefamandole sodium composition
[0033] The composition comprises: 1 part by weight of the cefamandole sodium crystal prepared by the present invention, and 0.001 part by weight of anhydrous sodium carbonate.
[0034] The preparation method is:
[0035] (1) Weigh cefamandole sodium crystals and anhydrous sodium carbonate in proportion and mix them thoroughly;
[0036] (2) Dispense into sterilized vials and stopper them.
Embodiment 3
[0037] Example 3: Preparation of cefamandole sodium composition
[0038] The composition comprises: 1 part by weight of the cefamandole sodium crystal prepared by the present invention, and 0.0015 part by weight of anhydrous sodium carbonate.
[0039] The preparation method is:
[0040] (1) Weigh cefamandole sodium crystals and anhydrous sodium carbonate in proportion and mix them thoroughly;
[0041] (2) Dispense into sterilized vials and stopper them.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com