Acyloxy containing hydroxyalkyl polysiloxane and preparation method
A technology containing acyloxy hydroxyalkyl polysiloxane and hydroxyalkyl methacrylate, which is applied in the field of organosiloxane synthesis and can solve the problems affecting the performance of modified polyacrylic acid and polyether, and the breaking of molecular chains. , to achieve the effect that the synthesis conditions are mild and not harsh, and the raw materials are convenient and easy to obtain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
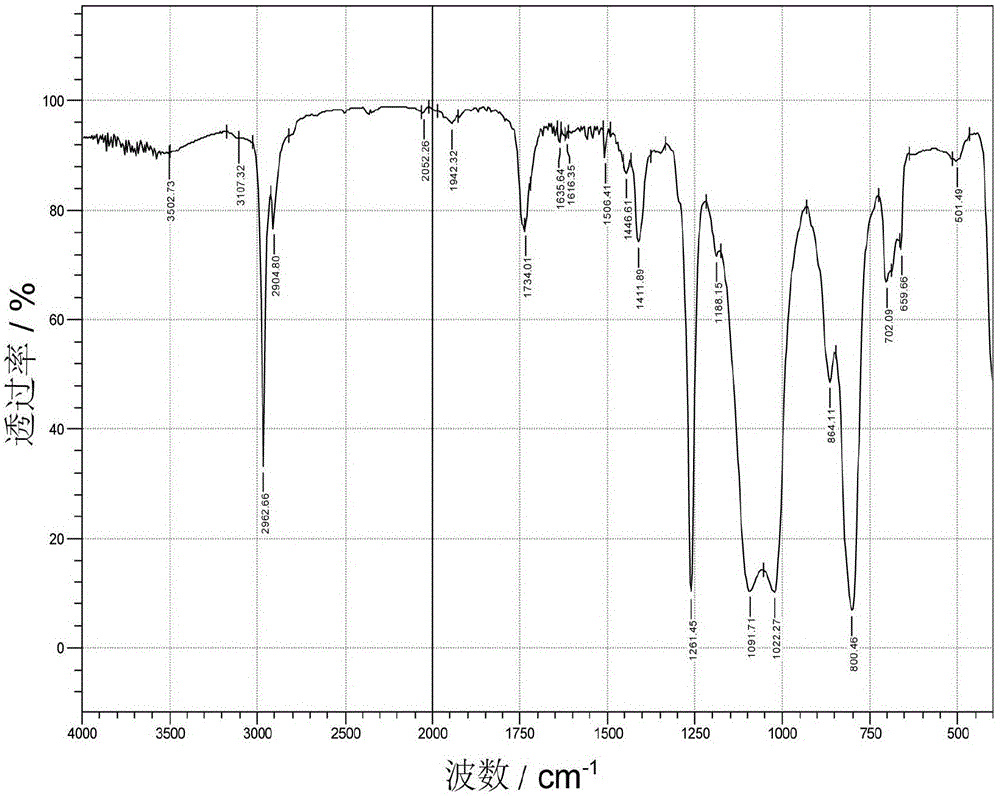
[0046] Add 100g terminal hydrogen-containing polysiloxane, 11.6g hydroxyethyl acrylate (C=C:Si—H molar ratio 1.0:1.0), 111.6g toluene ( 100% of the total mass of the reaction raw materials), 0.58g hydroquinone (0.05% of hydroxyethyl acrylate), through N 2 Replace three times, gradually heat up to 90°C, add 11.2g of 1wt% chloroplatinic acid-ethanol solution (378ppm) as a catalyst, the system heats up rapidly, after the system temperature is stable, gradually heat up to 95°C for 3 hours, and drop to At room temperature, wash with water three times to remove unreacted hydroxyethyl acrylate, after water separation, heat up toluene to reflux with water, then raise the temperature to 100°C, remove toluene under reduced pressure at -0.090MPa vacuum, cool down to room temperature, and filter to obtain terminal acyloxy Hydroxyethylpolysiloxane. The infrared spectra of hydrogen-terminated polysiloxane, hydroxyethyl acrylate and acyloxy-terminated hydroxyethyl polysiloxane are as follow...
Embodiment 2
[0048] Add 100g terminal hydrogen-containing polysiloxane, 26.0g hydroxypropyl acrylate (C=C:Si—H molar ratio 2.0:1.0), 63g xylene ( 50% of the total mass of the reaction raw materials), 0.003g hydroquinone (0.01% of hydroxyethyl acrylate), through N 2 Replaced three times, gradually warming up to 90°C, adding 0.334g of 1wt% chloroplatinic acid-isopropanol solution (10ppm), the system heated up rapidly, after the system temperature was stabilized, gradually warming up to 145°C for 5 hours, after the reaction was completed, it dropped to At room temperature, wash with water three times to remove excess hydroxypropyl acrylate, after separating water, raise the temperature to remove water, then raise the temperature to 100°C, remove xylene under reduced pressure at -0.090MPa vacuum, cool down to room temperature, and filter to obtain terminal acyloxyhydroxyethyl base polysiloxane. The infrared spectrum of acyloxy-terminated hydroxyethyl polysiloxane is as follows figure 2 show...
Embodiment 3
[0050] In a 250mL three-necked flask equipped with a thermometer, a stirrer and a condenser tube, add 100g of terminal hydrogen-containing polysiloxane, 26.0g of hydroxypropyl acrylate (C=C:Si—H molar ratio 2.0:1.0), 0.26g of p-phenylene Diphenol (1% of hydroxypropyl acrylate), thru N 2 Replace three times, gradually heat up to 90°C, add 33.46g of 1wt% chloroplatinic acid-isopropanol solution (1000ppm), the system heats up rapidly, after the system temperature is stable, gradually heat up to 100°C for 3 hours, and drop to At room temperature, wash with water three times to remove excess hydroxypropyl acrylate, add an appropriate amount of toluene to remove water after water separation, then raise the temperature to 100°C, remove toluene under reduced pressure at -0.090MPa vacuum, cool down to room temperature, and filter to obtain terminal acyl oxide Hydroxypropyl polysiloxane. The infrared spectrum of terminal acyloxy hydroxypropyl polysiloxane is as follows image 3 shown....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com