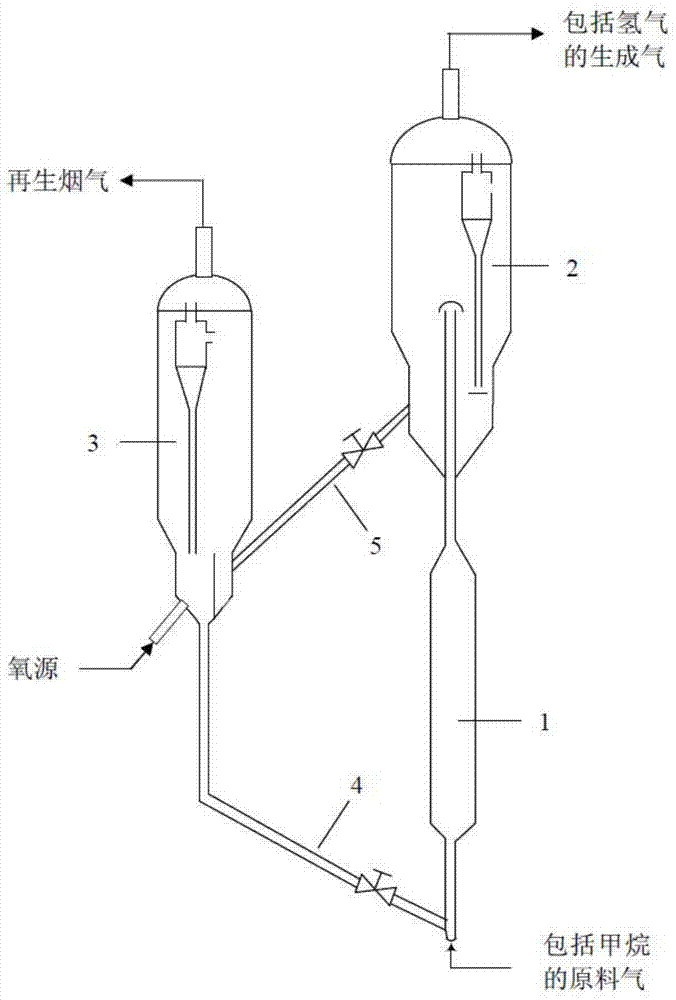
Nickel-based catalyst and preparation method thereof and method for producing hydrogen by catalytic cracking of methane
A nickel-based catalyst, a technology for producing hydrogen, which is applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. , the process is cumbersome and other problems, to achieve the effect of efficiently regenerating the catalyst, avoiding the deposition of carbon powder, and prolonging the service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] This embodiment provides a nickel-based catalyst that catalyzes the cracking of methane to produce hydrogen. The nickel-based catalyst is prepared by the following method:
[0084]Add 300.48g of deionized water to 78.25g of pseudo-boehmite, stir well with 80°C water bath, add hydrochloric acid to adjust the pH value to about 3-4, and obtain the gel of the carrier; mix 15.6g of cobalt nitrate, 16.2g Mix phosphotungstic acid into the prepared gel, then add 70g of deionized water, stir evenly, then add 100mL of 0.1mol / L potassium carbonate solution dropwise at a speed of 1mL / min, and dry at 150°C 24 hours, then roasted at 400°C for 18 hours, cooled, crushed and sieved to obtain a modified carrier; weigh 50g of the modified carrier and add it to an aqueous solution of 39.8g of nickel nitrate and 40g of deionized water, and add 100mL of it dropwise at a rate of 1mL / min 0.1mol / L potassium carbonate solution, washed and filtered the precipitate, then dried at 140°C for 12 hour...
Embodiment 2
[0097] This embodiment provides a nickel-based catalyst that catalyzes the cracking of methane to produce hydrogen. The nickel-based catalyst is prepared by the following method:
[0098] Add 300.48g deionized water and 127.64g silica sol to 48.97g pseudo-boehmite, stir well with 80°C water bath, add hydrochloric acid to adjust the pH value to about 3-4, and obtain the gel of the carrier; Cobalt nitrate and 16.2g of phosphotungstic acid were mixed and added to the prepared gel, then 70g of deionized water was added, and mechanically stirred evenly, and then 100mL of 0.1mol / L potassium carbonate solution was added dropwise at a speed of 1mL / min. Dry at 150°C for 24 hours, then roast at 400°C for 18 hours, crush and sieve after cooling to obtain a modified carrier; weigh 50g of the modified carrier and add it to an aqueous solution of 39.8g of nickel nitrate and 40g of deionized water, at 1mL / min Add 100mL of 0.1mol / L potassium carbonate solution dropwise at a high speed, wash a...
Embodiment 3
[0102] This embodiment provides a nickel-based catalyst that catalyzes the cracking of methane to produce hydrogen. The nickel-based catalyst is prepared by the following method:
[0103] Add 90.47g of deionized water to 209.87g of silica sol to obtain a gel of the carrier; mix 16.8g of copper nitrate, 7.7g of cerium nitrate, and 1.7g of niobium hydroxide into the prepared gel, and then add 70g of Deionized water, mechanically stirred evenly, then 100mL of 0.1mol / L potassium carbonate solution was added dropwise at a rate of 1mL / min, dried at 150°C for 24 hours, then roasted at 400°C for 18 hours, cooled, crushed and sieved to obtain Modified carrier: Weigh 50g of modified carrier and add 39.8g of nickel nitrate and 40g of deionized water in the aqueous solution, add 100mL of 0.1mol / L potassium carbonate solution dropwise at a speed of 1mL / min, wash and filter the precipitate, Dry at ℃ for 12 hours, then bake at 700℃ for 17 hours, after cooling, crush and sieve to obtain the c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com