Novel metformin hydrochloride solid medicinal preparation and preparation method thereof
A technology of metformin hydrochloride and solid pharmaceutical preparation, which is applied in the field of new solid pharmaceutical preparation of metformin hydrochloride and its preparation field, can solve the problems of increasing the occurrence of adverse reactions, being difficult to improve bioavailability, discounting the treatment effect, etc., and reducing the occurrence of adverse reactions. , increase bioavailability, exert therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
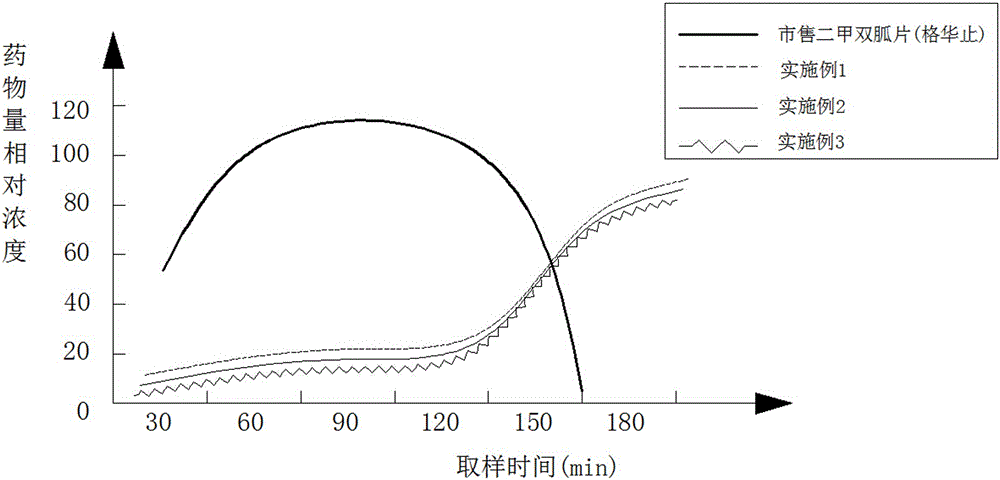
Image
Examples
Embodiment 1
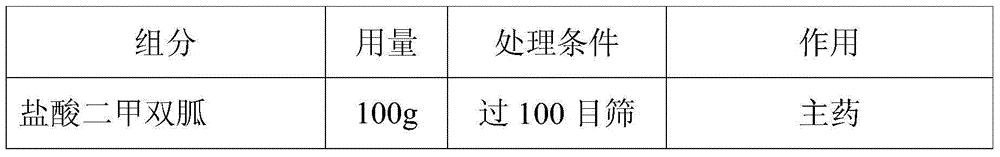
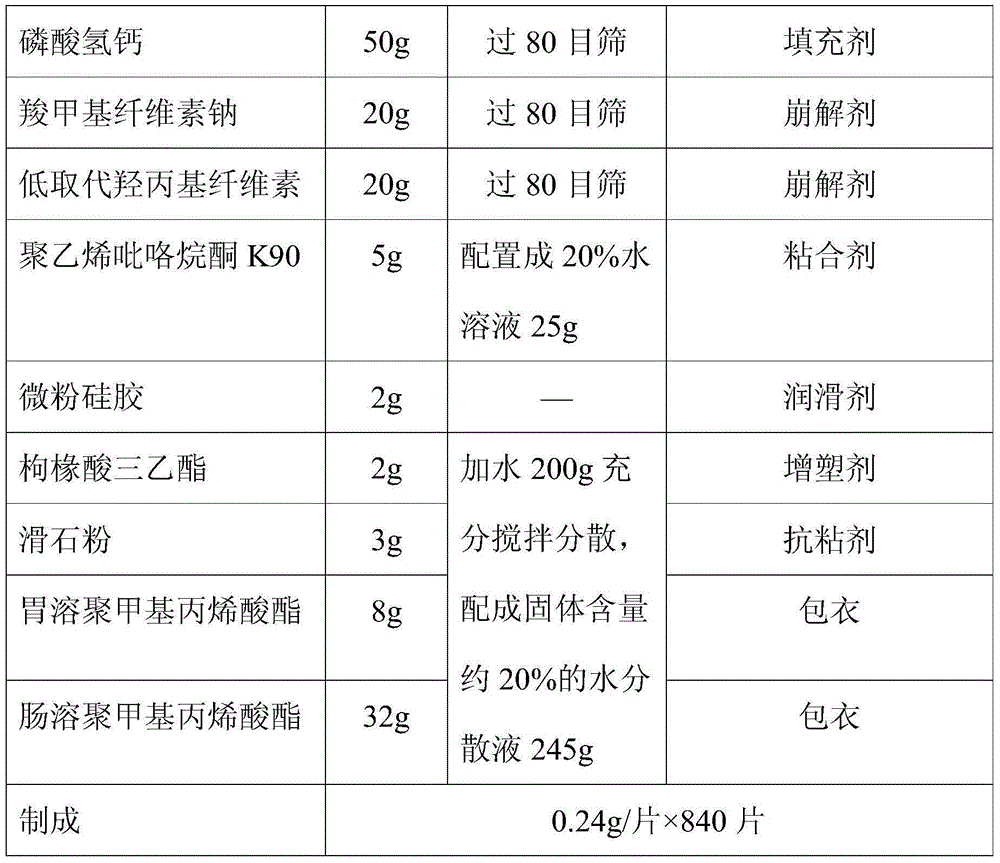
[0028] The preparation method of a novel metformin hydrochloride solid pharmaceutical preparation of the present embodiment is prepared by a known method in the pharmaceutical industry, and the specific dosage of each component is shown in Table 1 below:
[0029] Table I
[0030]
[0031]
[0032] The specific preparation method is preparation, after mixing metformin hydrochloride, calcium hydrogen phosphate, sodium carboxymethyl cellulose, and low-substituted hydroxypropyl cellulose evenly, adding to an aqueous solution of polyvinylpyrrolidone K90, and stirring to make a soft material.
[0033] The soft material is made into wet granules through 18 sieves, and the wet granules are dried in an environment of 50-60°C until the moisture content of the wet granules is 3%-5%, then add micro-powdered silica gel and mix evenly, press 0.2g / tablet Formed into tablets, this is the plain tablet to be coated.
[0034] Add stomach-soluble polymethacrylate, enteric-coated polymethac...
Embodiment 2
[0037] The preparation method of a novel metformin hydrochloride solid pharmaceutical preparation of this embodiment is prepared by a known method in the pharmaceutical industry, and the specific dosage of each component is shown in Table 2 below:
[0038] Table II
[0039]
[0040] Concrete preparation method is with embodiment 1, and concrete coating parameter is:
[0041] Tablet bed temperature: 25-35°C; air outlet temperature: 33-40°C; liquid spray speed: 3-6g / min / kg, that is, 2.4-5g / min; coating weight gain: 15%.
Embodiment 3
[0043] The preparation method of a novel metformin hydrochloride solid pharmaceutical preparation of this embodiment is prepared by a known method in the pharmaceutical industry, and the specific dosage of each component is shown in Table 3 below:
[0044] Table three
[0045]
[0046] Concrete preparation method is the same as above-mentioned embodiment, and concrete coating parameter is:
[0047] Tablet bed temperature: 25-35°C; air outlet temperature: 33-40°C, liquid spray speed: 3-6g / min / kg, ie 3.75-7.5g / min, coating weight gain: 10%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com