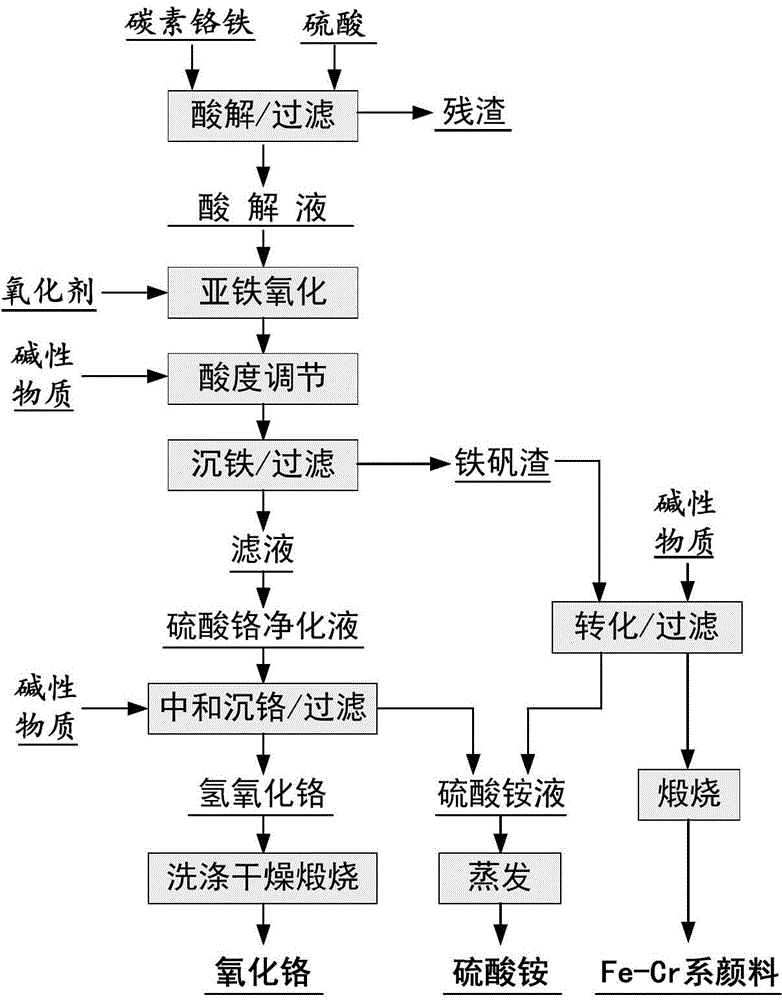
Method for producing chromic oxide from carbon ferrochrome
A technology of chromium trioxide and carbon ferrochromium, which is applied in chromium oxide/hydrate, chemical instruments and methods, iron compounds, etc. The effect of iron removal depth, high efficiency separation and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Accurately weigh 100g carbon ferrochrome and 201g concentrated sulfuric acid, and dilute the concentrated sulfuric acid into a sulfuric acid solution with a sulfuric acid mass fraction of 65%; measure half of the above sulfuric acid solution into an acidolysis tank made of glass, and heat the sulfuric acid solution to At about 90°C, slowly add the weighed ferrochrome carbon to make it slowly react with the sulfuric acid solution. After the slurry does not bubble, slowly dilute the slurry with water, and then separate the liquid and solid to remove the filter residue to obtain a mixed acid leaching solution of chromium sulfate and ferrous sulfate, in which the concentration of chromium is 20g / L; 2+ All oxidized to Fe 3+ , and adjust the pH value of the solution to 1.0 with ammonia water; then the Fe 2+ The chromium sulfate solution after oxidation and acidity adjustment is placed in an acid-resistant pressure kettle, and the temperature is raised to 160°C without adding...
Embodiment 2
[0031] Accurately weigh 100g carbon ferrochrome and 201g concentrated sulfuric acid, and dilute the concentrated sulfuric acid into a sulfuric acid solution with a mass fraction of sulfuric acid of 35%; measure half of the above sulfuric acid solution into an acidolysis tank made of glass, and heat the sulfuric acid solution to At about 140°C, slowly add the weighed ferrochrome carbon to make it slowly react with the sulfuric acid solution. After the slurry does not bubble, slowly dilute the slurry with water, and then separate the liquid and solid to remove the filter residue to obtain a mixed acid leaching solution of chromium sulfate and ferrous sulfate, in which the concentration of chromium is 120g / L; 2+ mostly oxidized to Fe 3+ , and use hydrogen peroxide to further convert Fe 2+ Oxidation is complete, then adjust the pH value of the solution to 3.2 with ammonium bicarbonate; 2+ The chromium sulfate solution after oxidation and acidity adjustment is placed in an acid-r...
Embodiment 3
[0033] Accurately weigh 100g carbon ferrochrome and 201g concentrated sulfuric acid, and dilute the concentrated sulfuric acid into a sulfuric acid solution with a mass fraction of sulfuric acid of 50%; measure half of the above sulfuric acid solution into an acidolysis tank made of glass, and heat the sulfuric acid solution to At about 115°C, slowly add the weighed ferrochrome carbon to make it slowly react with the sulfuric acid solution. After the slurry does not bubble, slowly dilute the slurry with water, and then separate the liquid and solid to remove the filter residue to obtain a mixed acid leaching solution of chromium sulfate and ferrous sulfate, in which the concentration of chromium is 50g / L; 2+ All oxidized to Fe 3+ , and then adjust the pH value of the solution to 2.65 with ammonia water; then the Fe 2+ The chromium sulfate solution after oxidation and acidity adjustment is placed in an acid-resistant pressure kettle, and the amount of seed crystals added is 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com