Nitrogen-doped porous carbon sphere and cobaltous oxide nano-composite anode material based on chitosan and derivatives thereof and preparation method thereof
A technology of nitrogen-doped porous carbon and nanocomposite materials, applied in battery electrodes, electrochemical generators, electrical components, etc., to achieve good conductivity, prevent agglomeration, and increase the effect of contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
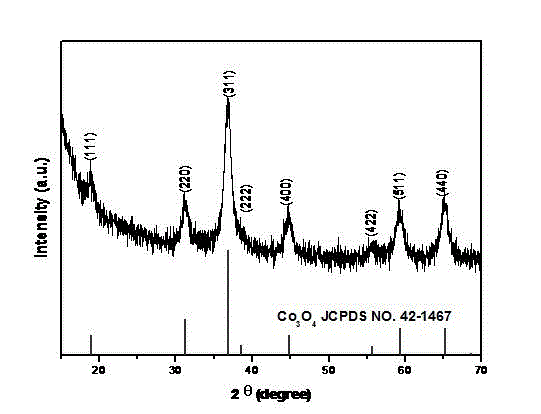
[0033] Mix 0.32g of cetyltrimethylammonium bromide, 2ml of tetraethyl orthosilicate and 2ml of ammonia (NH 3The mass percentage is 25~28%) was added to 106ml of deionized water and 56ml of absolute ethanol mixed solvent and stirred for 8h; at the same time, carboxymethyl chitosan was dissolved in deionized water to form 100ml of a solution with a mass percentage of 5%. The above solution was mixed and stirred for 20 hours; then the solvent of the mixed solution was evaporated and put into a drying oven at 100°C for solidification treatment for 24 hours. After the solid material was ground, it was carbonized under the protection of high-purity nitrogen. The carbonization temperature was 800°C and the carbonization time was 4h, the heating rate is 5°C / min; the carbonized product is fully stirred at room temperature for 20h with 5% hydrofluoric acid solution by mass percent, then washed with deionized water for 3 times, and dried at 80°C to obtain nitrogen-doped porous carbon bal...
Embodiment 2
[0037] Mix 0.36g cetyltrimethylammonium bromide, 3ml tetraethyl orthosilicate and 3ml ammonia water (NH 3 content of 25~28%) was added to 116ml of deionized water and 60ml of anhydrous ethanol mixed solvent and stirred for 6h; at the same time, carboxylated chitosan was dissolved in deionized water to form 100ml of a solution with a mass percentage of 6%, and then mixed with the above solution Mix and stir for 18 hours; then evaporate the solvent of the mixed solution and put it into a drying oven at 100°C for solidification treatment for 20 hours. After the solid material is ground, it is carbonized under the protection of high-purity nitrogen. The carbonization temperature is 700°C and the carbonization time is 2 hours. The heating rate was 10°C / min; the carbonized product was fully stirred at room temperature for 18 hours with a 10% by mass hydrofluoric acid solution, washed three times with deionized water, and dried at 80°C to obtain nitrogen-doped porous carbon spheres. ...
Embodiment 3
[0041] Mix 0.32g of cetyltrimethylammonium bromide, 2ml of tetraethyl orthosilicate and 2ml of ammonia (NH 3 content of 25~28%) was added to 106ml of deionized water and 56ml of anhydrous ethanol mixed solvent and stirred for 8 hours; at the same time, 300,000 molecular weight chitosan was dissolved in acetic acid aqueous solution with a volume concentration of 2% to form 80ml with a mass percentage of 4 % solution and mixed with the above solution for 24 hours; then the solvent of the mixed solution was evaporated and placed in a drying oven at 120°C for solidification treatment for 20 hours. ℃, the carbonization time is 3h, and the heating rate is 5°C / min; the carbonized product is fully stirred at room temperature for 20h with an 8% hydrofluoric acid solution by mass percent, washed three times with deionized water, and dried at 100°C to obtain Nitrogen-doped porous carbon spheres.
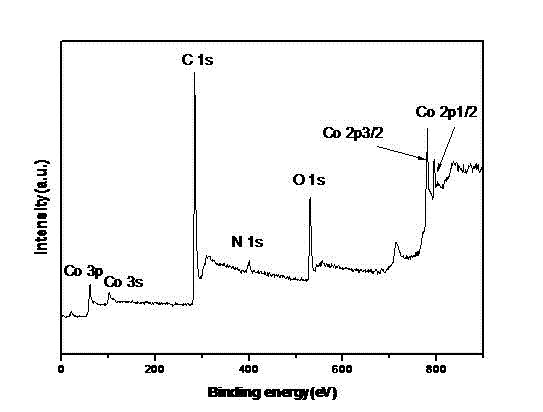
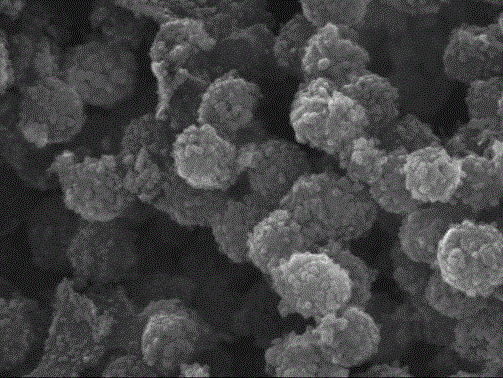
[0042] Weigh 80 mg of nitrogen-doped porous carbon spheres and add them to 100 ml of absol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com