Determination method for content of mussaendoside G in medicinal material Mussaenda pubescens
A kind of technology of golden anthocyanidin and golden flower, applied in the field of content determination of golden anthoside G and golden flower of golden leaf in medicinal materials, can solve the problems such as no content determination, achieve good reproducibility, accurate measurement result, high precision effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
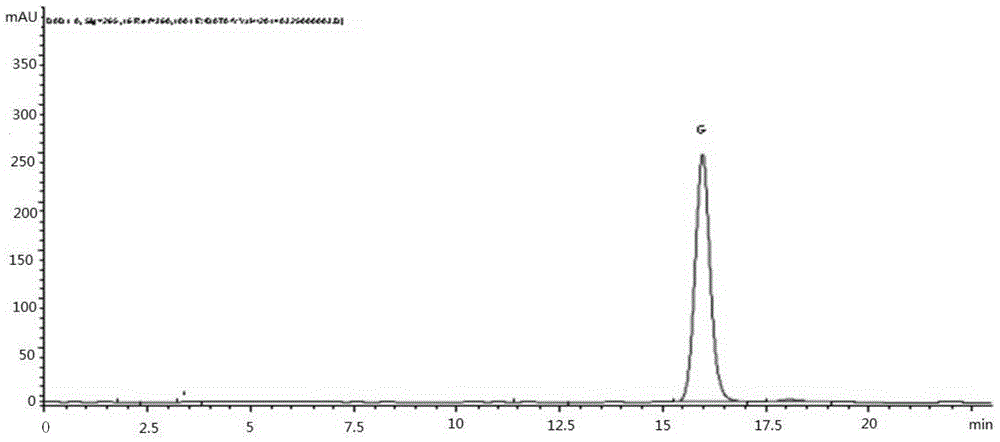
[0061] Example 1 Determination of the content of aureoside G in Jade japonica medicinal material (National Inspection Institute reference medicinal material 121404-200401)
[0062] According to the "Chinese Pharmacopoeia" 2010 edition an appendix VID high performance liquid chromatography determination operation, the steps are as follows:
[0063] Chromatographic conditions and system suitability experiment
[0064] Use C18 bonded silica gel as the filler; use acetonitrile:water=38:62 as the mobile phase; the flow rate is 1ml min -1 ;The column temperature is 25°C; the detection wavelength is 265nm; the number of theoretical plates should not be less than 3000;
[0065] Preparation of reference solution
[0066] Accurately weigh an appropriate amount of the reference substance of euphyllin G, and make 0.60mg·ml with methanol -1 solution;
[0067] Preparation of the test solution
[0068] Take 3.0g of Yuye Jinhua medicinal material powder (passing 40 mesh), weigh it acc...
Embodiment 2
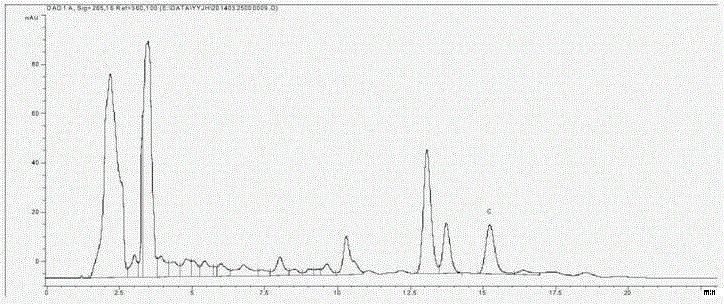
[0072] Determination of the content of Jade leaf aureoside G in embodiment 2 Jade leaf golden flower medicinal material (Bobai County, Guangxi)
[0073] According to the "Chinese Pharmacopoeia" 2010 edition an appendix VID high performance liquid chromatography determination operation, the steps are as follows:
[0074] Chromatographic conditions and system suitability experiment
[0075] Use C18 bonded silica gel as the filler; use acetonitrile:water=35:65 as the mobile phase; the flow rate is 1ml min -1 ;The column temperature is 30°C; the detection wavelength is 268nm; the number of theoretical plates should not be less than 3000;
[0076] Preparation of reference solution
[0077] Accurately weigh an appropriate amount of the reference substance of euphyllin G, and make 0.06mg·ml with methanol -1 solution;
[0078] Preparation of the test solution
[0079] Take 2.0g of Yuye Jinhua medicinal material powder (passing 40 mesh), weigh it accurately, put it in a stopper...
Embodiment 3
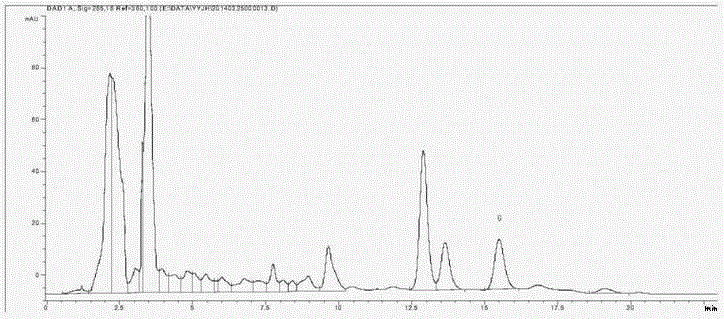
[0083] Determination of the content of euphyllin G in embodiment 3 euphyllum japonica medicinal material (Jinxiu County, Guangxi)
[0084] According to the "Chinese Pharmacopoeia" 2010 edition an appendix VID high performance liquid chromatography determination operation, the steps are as follows:
[0085] Chromatographic conditions and system suitability experiment
[0086] Use C18 bonded silica gel as the filler; use acetonitrile:water=25:75 as the mobile phase; the flow rate is 1ml min -1 ;The column temperature is 30°C; the detection wavelength is 270nm; the number of theoretical plates should not be less than 3000;
[0087] Preparation of reference solution
[0088] Accurately weigh an appropriate amount of the reference substance of euphyllin G, and make 0.30mg·ml with methanol -1 solution;
[0089] Preparation of the test solution
[0090] Take 5.0g of Yuye Jinhua medicinal material powder (passing 40 mesh), weigh it accurately, put it in a stoppered Erlenmeyer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com