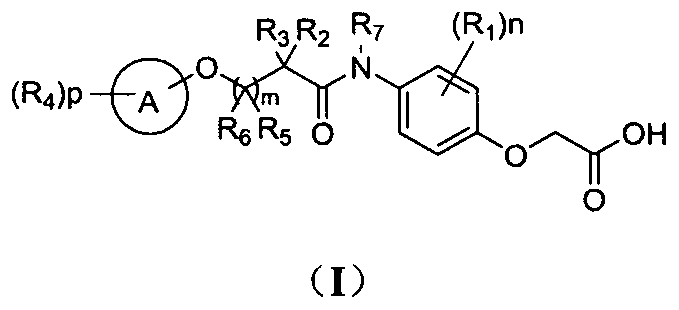
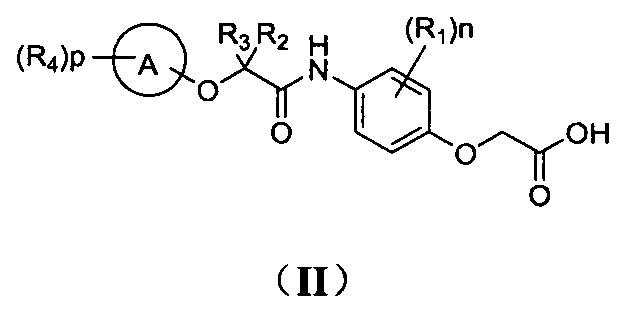
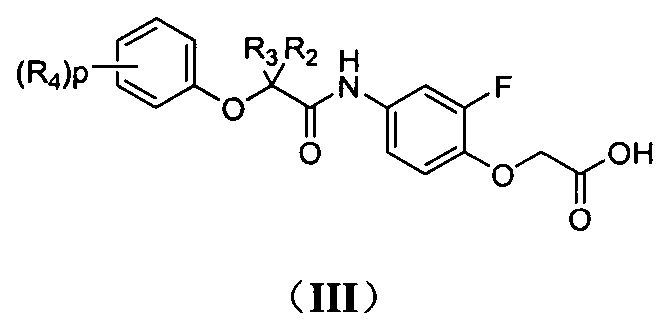
Phenoxyacetic acid derivatives and preparation method thereof, and application of phenoxyacetic acid derivatives as drug
A technology of acetic acid and alkoxy, applied in the field of pharmacy, can solve the problems of hypoglycemia and weight gain, side effects, edema side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] 2-(2-fluoro-4-(2-phenoxyacetylamino)phenoxy)acetic acid (I-1)
[0103]
[0104] first step
[0105] Methyl 2-(4-(2-chloroacetylamino)-2-fluorophenoxy)acetate (1c)
[0106] Methyl 2-fluoro-4-aminophenoxyacetate 1b (2.0 g, 10.1 mmol) was dissolved in CH 2 Cl 2 (50mL), add triethylamine (2.7mL, 20.2mmol) and a catalytic amount of DMAP, cool to 0°C, slowly drop CH 2 Cl 2 Dissolved chloroacetyl chloride 1a (1.5mL, 15.1mmol), reacted at 0°C for 2h, after the reaction was complete by TLC, quenched the reaction by adding water, CH 2 Cl 2 (30ml×4) extraction, the combined organic phases were sequentially washed with 1N NaOH (20ml×1), 1N HCl (20ml×1), saturated NaCl solution (20ml×2), dried, concentrated, and recrystallized with EtOH to obtain 2.2g of a white solid , the yield was 75.9%.
[0107] second step
[0108] Methyl 2-(2-fluoro-4-(2-phenoxyacetamido)phenoxy)acetate (1d)
[0109] Methyl 2-(4-(2-chloroacetamido)-2-fluorophenoxy)acetate 1c (0.20 g, 0.73 mmol) and...
Embodiment 2
[0115] 2-(2-fluoro-4-(2-(o-tolyloxy)acetamide)phenoxy)acetic acid (I-2)
[0116]
[0117] Referring to the preparation method of I-1, 103 mg of white solid was obtained, the yield was 50.4%, and mp: 172-174°C.
[0118] 1 H NMR (300MHz, DMSO-d 6 )δ: 13.02(s, 1H), 10.13(s, 1H), 7.62, 7.58(dd, J=1.8, 13.6Hz, 1H), 7.28(d, J=7.4Hz, 1H), 7.15-7.05(m , 3H), 6.87-6.84(m, 2H), 4.73(s, 2H), 4.68(s, 2H), 2.24(s, 3H); 13 C NMR (75MHz, DMSO-d 6 )δ: 169.84, 166.63, 155.95, 152.44, 141.55, 132.32, 132.19, 126.87, 126.16, 120.94, 118.23, 115.45, 112.13, 109.36, 67.36, 65.24, 16.06 (: ESI-MS2 m / z] - ).
Embodiment 3
[0120] 2-(2-Fluoro-4-(2-(m-tolyloxy)acetamide)phenoxy)acetic acid (I-3)
[0121]
[0122] Referring to the preparation method of I-1, 115 mg of white solid was obtained, the yield was 47.1%, and mp: 157-159°C.
[0123] 1 H NMR (300MHz, DMSO-d 6 )δ: 13.09(s, 1H), 10.09(s, 1H), 7.62, 7.58(dd, J=1.8, 13.6Hz, 1H), 7.31(d, J=8.6Hz, 1H), 7.18(t, J =8.6Hz, 1H), 7.05(t, J=9.3Hz, 1H), 6.83-6.78(m, 3H), 4.73(s, 2H), 4.64(s, 2H), 2.27(s, 3H); 13 C NMR (75MHz, DMSO-d 6 ( - ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com