Preparation and application of mitochondrial fluorescent dye 3-heteroaryl substituted-2h-indazole derivatives
A heteroaryl and indazole-based technology, applied in the field of fluorescent compounds, can solve problems such as poor tolerance of functional groups, difficulty in obtaining raw materials, harsh reaction conditions, etc., and achieve the effects of small light damage, improved sensitivity, and small molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Synthesis of 3-(benzothienyl-2-)2-methyl-2H-indazole
[0041] (1) 2-methyl-2H-indazole (33mg, 0.25mmol), benzothiophene (101mg, 0.75mmol), tetrakis (triphenylphosphine) palladium (15mg, 0.0125mmol), pyridine (20mg, 0.25 mmol) and 1,4-dioxane (0.5mL) were added to the reaction tube, stirred evenly under anhydrous and oxygen-free conditions, heated to 120°C, and reacted for 24 hours;
[0042] (2) After the reaction is completed, cool the reaction tube to room temperature, add 10 mL of dichloromethane to dilute the reaction system, then filter through diatomaceous earth and wash with 10-20 mL of dichloromethane, combine the filtrates, remove the solvent under reduced pressure, and the remaining The product was separated and purified by silica gel column chromatography (dichloromethane / petroleum ether / ethyl acetate=2:7:1, v / v / v), and after vacuum drying, the target product 3-(benzothienyl-2 -) 2-methyl-2H-indazole 44 mg, yield 67%. 1 H NMR (400MHz, CDCl 3 ):δ=...
Embodiment 2
[0043] Example 2: Synthesis of 2-methyl-3-(5-methoxythienyl-2-)-5-methoxy-2H-indazole
[0044] (1) Mix 2-methyl-5-methoxy-2H-indazole (40mg, 0.25mmol), 2-methoxythiophene (76μL, 0.75mmol), tetrakis(triphenylphosphine) palladium (15mg, 0.0125mmol), pyridine (20mg, 0.25mmol) and 1,4-dioxane (0.5mL) were added to the reaction tube, stirred evenly under anhydrous and oxygen-free conditions, heated to 120°C, and reacted for 24 hours;
[0045] (2) After the reaction is completed, cool the reaction tube to room temperature, add 10 mL of dichloromethane to dilute the reaction system, then filter through diatomaceous earth and wash with 10-20 mL of dichloromethane, combine the filtrates, remove the solvent under reduced pressure, and the remaining The product was separated and purified by silica gel column chromatography (petroleum ether / ethyl acetate=3:1, v / v), and after vacuum drying, the target product 2methyl-3-(5-methoxythienyl-2- )-5-methoxy-2H-indazole 36 mg, yield 52%. 1 H NM...
Embodiment 3
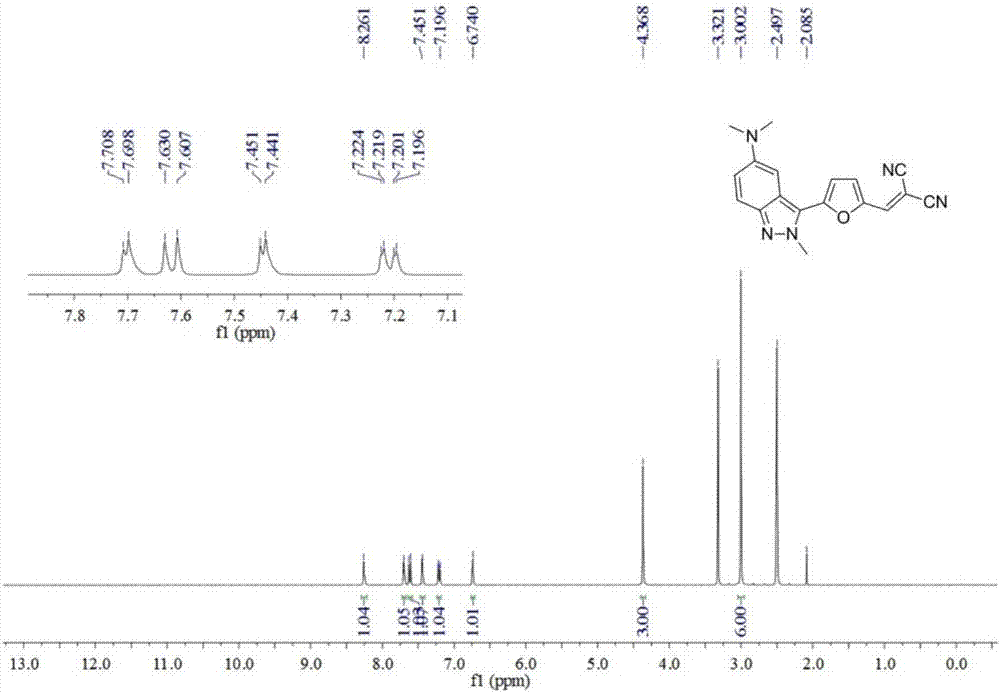
[0046] Example 3: Synthesis of 2-((5-(5-(dimethylamino)-2-methyl-2H-indazolyl-3-)furan-2-)methylene)malononitrile
[0047] (1) Add N,N,2-trimethyl-2H-indazol-5-amine (44 mg, 0.25 mmol), furan-2-carbaldehyde (62 μL, 0.75 mmol), tetrakis(triphenylphosphine) palladium ( 15mg, 0.0125mmol), pyridine (20mg, 0.25mmol) and 1,4-dioxane (0.5mL) were added to the reaction tube, stirred evenly under anhydrous and oxygen-free conditions, heated to 120°C, and reacted for 24 hours;
[0048] (2) After the reaction is completed, cool the reaction tube to room temperature, add 10 mL of dichloromethane to dilute the reaction system, then filter through diatomaceous earth and wash with 10-20 mL of dichloromethane, combine the filtrates, remove the solvent under reduced pressure, and the remaining The product was separated and purified by silica gel column chromatography (dichloromethane / petroleum ether / ethyl acetate=1:3:1, v / v / v), and after vacuum drying, a red solid 5-(5-(dimethylamino)- 2-Meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com