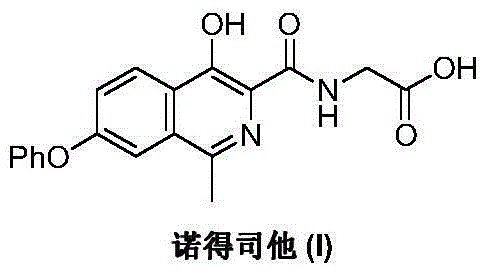
Preparation method of Roxadustat
A technology of methyl and formate esters, which is applied in the field of preparation of the drug Nordrestat, can solve problems such as difficult acquisition of enlightening raw materials, difficulty in industrial production, and cumbersome preparation process, and achieve the goal of promoting development, easy access to raw materials, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
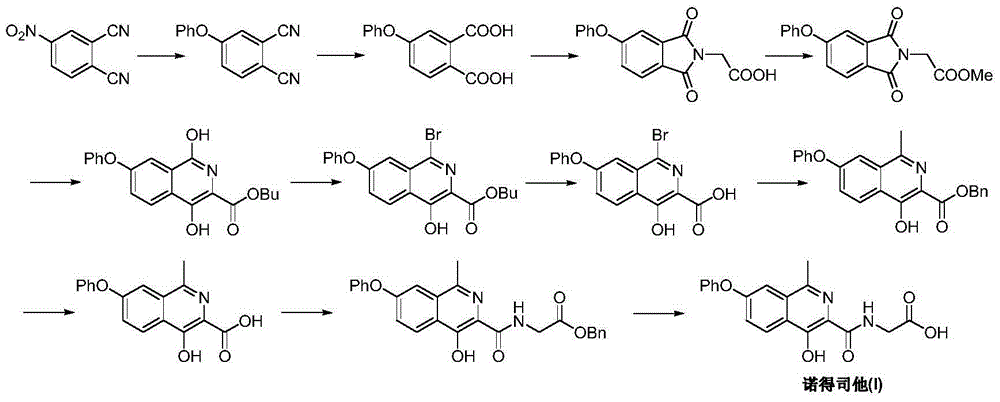
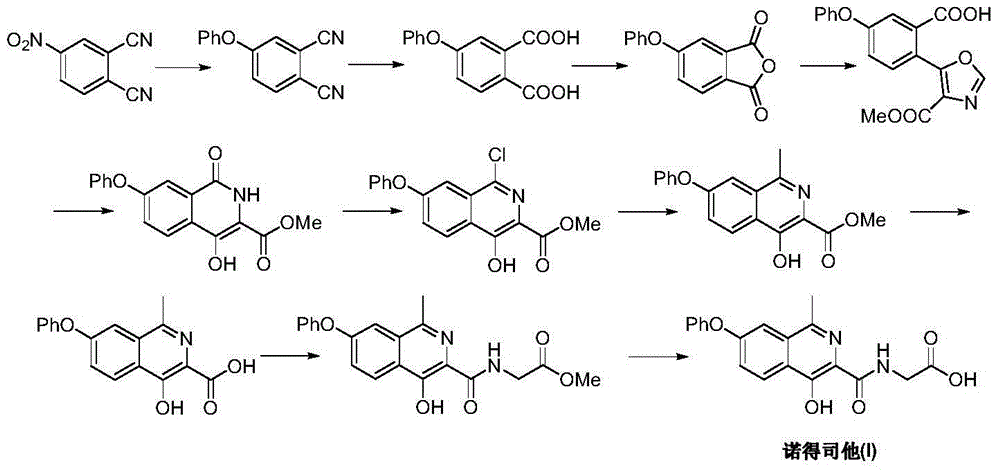
Image
Examples
Embodiment 1
[0039] Add tyrosine (18.1 g, 0.1 mmol) and 250 mL of methanol into the dry reaction flask, cool down to 0-5° C. in an ice bath, and add 10 g of concentrated sulfuric acid with a weight percentage of 98% dropwise within 1 hour. After dropping, the temperature was raised to reflux. Stir the reaction for 16-20 hours, and TLC detects that the reaction is complete. Concentrate under normal pressure, add 100mL of pure water to the residue, adjust the pH to 6.5-7.0 with 10% by weight sodium hydroxide, a solid precipitates, filter, wash the chlorine cake with methanol and water (1:1), and vacuum dry to obtain white Solid tyrosine methyl ester (II) 15.3g, yield 78.5%; EI-MS m / z: 196[M+H] + .
Embodiment 2
[0041] In nitrogen atmosphere and ice bath, add tyrosine methyl ester (II) (9.8g, 50mmol), potassium methoxide (3.5g, 50mmol) and methanol 50mL in the reaction flask, after no gas is generated, heat to reflux, stir React for 2 hours. Concentrate under normal pressure to remove the solvent, add 25 mL of dimethyl sulfoxide and freshly prepared copper powder (0.2 g, 3.1 mmol) to the residue, and slowly heat up to 150-155 °C under stirring. After about half an hour, add bromobenzene (7.9 g , 50mmol), continue to heat up to 170-175°C, stir and react for 3 hours, and TLC detects that the reaction is complete. Cool to 60°C, add methanol and keep boiling slightly, filter while hot, wash the filter cake three times with hot ethanol, combine the organic phases, cool to 0°C, filter, and dry in vacuo to obtain a white solid 2-amino-3-(4- Methyl phenoxyphenyl)propionate (III) 11.5g, yield 84.9%; EI-MS m / z: 272[M+H] + .
Embodiment 3
[0043] Add 2-amino-3-(4-phenoxyphenyl) methyl propionate (III) (10.8g, 40mmol), 40% by weight acetaldehyde (20g, 0.2mol) and weight percent in reaction flask 50 mL of 35% concentrated hydrochloric acid was refluxed for 1 hour. Continue to add 40% by weight acetaldehyde (10 g, 0.1 mol) and 25 mL of concentrated hydrochloric acid with 35% by weight, and then reflux for 3-5 hours. Cool to 4-7 ° C, add ethyl acetate, extract and separate layers. The aqueous layer was adjusted to pH 11-12 with sodium hydroxide solution, extracted three times with ethyl acetate, the organic phases were combined, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain a white solid 1-methyl-3-methyl-3-formate-7- Phenoxy-1,2,3,4-tetrahydroisoquinoline (IV) 8.4g, yield 70.7%; mass spectrum (EI): EI-MS m / z: 298[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com