Children pharmaceutical composition containing ceftazidime and low-sodium carrier
A technology of ceftazidime and its composition, which is applied in the field of medicinal chemistry, can solve the problems of many impurities, obvious side effects, and low purity of active ingredients, and achieve the effects of low impurities, improved solubility, and improved clinical application quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
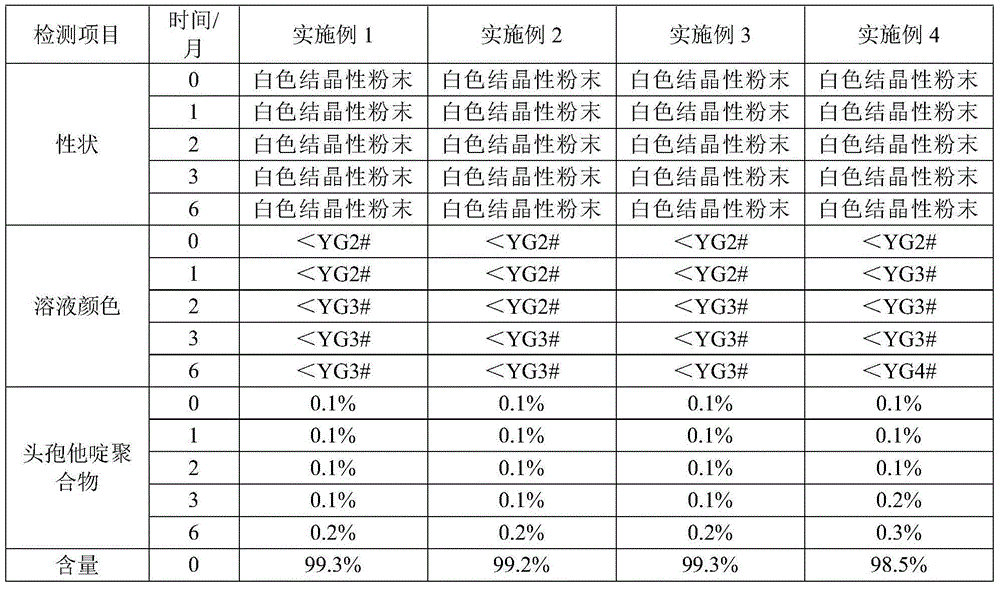
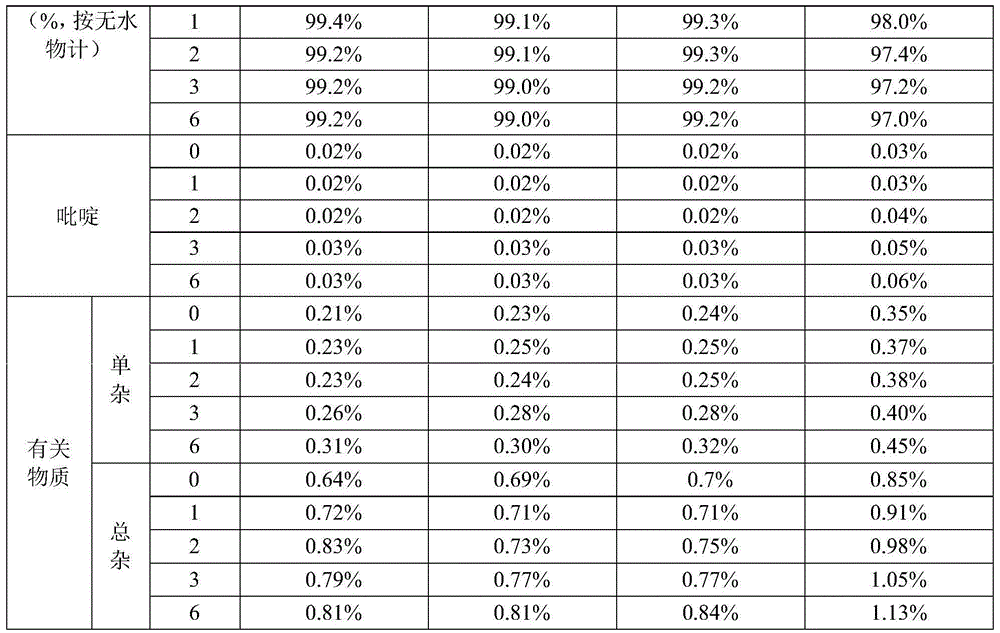
Examples
Embodiment 1
[0037] (1) Control the temperature at 10-15°C, weigh 100g of ceftazidime dihydrochloride crude product, add 1000ml of water, stir until completely dissolved, add 10g of activated carbon, stir for decolorization, and filter;
[0038] (2) Control the temperature at 0°C to 5°C, add 20% sodium hydroxide dropwise to adjust the pH to 6.2±0.2, add 10ml of ethyl acetate, stir slowly for 5 hours, pass through an organic filter membrane and transfer to a 1000ml pressure-resistant container to ensure that it is full And remove the air bubbles, seal the container, shake, and freeze at -18°C for 8 hours before taking it out;
[0039] (3) Liquid-solid separation, after the ice melts, transfer to a crystallization tank, control the temperature at 0°C to 5°C and stir slowly, add hydrochloric acid to adjust the pH to 2.5±0.2, and react for 10 hours; add 5000ml of acetone dropwise, Stir at a slow speed for 30 minutes, continue to grow crystals for 1 hour, filter with suction, wash with acetone,...
Embodiment 2
[0041] (1) Control the temperature at 10-15°C, weigh 100g of ceftazidime dihydrochloride crude product, add 1000ml of water, stir until completely dissolved, add 10g of activated carbon, stir for decolorization, and filter;
[0042] (2) Control the temperature at 0°C to 5°C, add 20% sodium carbonate dropwise to adjust the pH to 6.0±0.2, add 10ml of chloroform, stir slowly for 5 hours, pass through an organic filter membrane and transfer to a 1000ml pressure-resistant container to ensure that it is full and the air bubbles are removed , seal the container, oscillate, and freeze at -18°C for 8 hours before taking it out;
[0043] (3) Liquid-solid separation, after the ice melts, transfer to a crystallization tank, control the temperature at 0°C to 5°C and stir slowly, add hydrochloric acid to adjust the pH to 2.5±0.2, and react for 10 hours; add 5000ml of acetone dropwise, Stir at a slow speed for 30 minutes, continue to grow crystals for 1 hour, filter with suction, wash with a...
Embodiment 3
[0045] (1) Control the temperature at 10-15°C, weigh 100g of ceftazidime dihydrochloride crude product, add 1000ml of water, stir until completely dissolved, add 10g of activated carbon, stir for decolorization, and filter;
[0046] (2) Control the temperature at 0°C to 5°C, add 20% sodium hydroxide dropwise to adjust the pH to 6.3±0.2, add 7ml of ethyl acetate, stir slowly for 5 hours, pass through an organic filter membrane and transfer to a 1000ml pressure-resistant container to ensure that it is full And remove the air bubbles, seal the container, shake, and freeze at -18°C for 8 hours before taking it out;
[0047] (3) Liquid-solid separation, after the ice melts, transfer to a crystallization tank, control the temperature at 0°C to 5°C and stir slowly, add hydrochloric acid to adjust the pH to 2.5±0.2, and react for 10 hours; add 5000ml of acetone dropwise, Stir at a slow speed for 30 minutes, continue to grow crystals for 1 hour, filter with suction, wash with acetone, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com