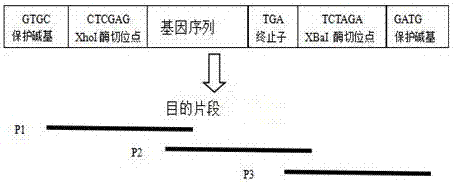
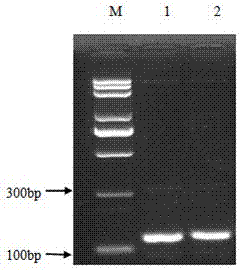
A kind of high-efficiency preparation method of antibacterial peptide cath-bf30 of golden snake
An antibacterial peptide, -cath-bf30 technology, which is applied in the field of efficient preparation of the antibacterial peptide Cath-BF30, can solve the problems of difficult synthesis, low yield of antibacterial peptide Cath-BF30, and difficulty in popularization and application. To achieve the effect of ensuring correct secretion and expression, good industrial performance, and clear genetic background
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0074]Example 2 Construction of the cloning vector of Cath-BF30 antimicrobial peptide
[0075] 1. The genetic engineering operation was carried out according to the molecular cloning experiment guide method, and the Cath-BF30 target gene synthesized by PCR and the vector plasmid PGAPZa were respectively used xho I and Xba I double digestion, and then ligation transformation. The specific procedure is as follows:
[0076] (1) Obtaining the vector plasmid PGAPZa: Cultivate Escherichia coli containing the PGAPZa plasmid overnight at 37°C and 170 rpm, extract the plasmid with a plasmid mini-extraction kit, perform gel electrophoresis, and store at -20°C.
[0077] (2) Double digestion: The vector plasmid PGAPZa and the Cath-BF30 gene were respectively digested with xho I and Xba I double enzyme digestion, 37 ℃ water bath for 2 hours, gel electrophoresis, observe whether the double enzyme digestion is complete, if the target fragment and the carrier have been double enzyme...
Embodiment 3
[0082] Example 3 Construction of engineering bacteria SMD-pGAPZα-30
[0083] 1. Linearization of recombinant expression plasmid pGAPZα-Cath-BF30
[0084] A large number of pGAPZα-Cath-BF30 plasmids were extracted with the plasmid extraction kit, and the Bln I enzyme linearizes it. 200μL linearization system: 10μL BlnI , 40 μL pGAPZα-Cath-BF30 (about 20 μg), 20 μL 10× buffer, 130 μL ddH 2 O. After mixing well, digest overnight at 37°C, take 2 μL of the digested product and do 1% agarose gel electrophoresis to observe whether it is completely linearized.
[0085] 2. Extract the fully linearized plasmid with phenol-chloroform to reach the concentration required for electroporation. The specific steps are as follows:
[0086] (1) Add 300 μL ddH to 200 μL enzyme digestion system 2 O to make a total volume of 500 μL.
[0087] (2) Add 500 μL of phenol chloroform to extract once, 12000 rpm, 10 min, carefully take the upper layer in the stratification.
[0088] (3) Add 1 / 10 ...
Embodiment 4
[0138] Example 4 Fermentation of engineering bacteria SMD-pGAPZα-30
[0139] 1. Observation of the effect of pH value on the secretion and expression of Cath-BF
[0140] Performed at the shake flask level, adjust the pH to 4.0, 5.0, 6.0 with phosphate buffer, and adjust the pH 6.8 YPD medium without phosphate buffer, inoculate the engineered bacteria SMD-pGAPZα- at 1% (v / v) 30 seed liquids, 28°C, 170rpm shaking culture for 96h, take 0h, 24h, 48h, 72h, 96h samples, measure the corresponding pH, cell density OD 600 and the size of the inhibition zone to determine the optimum pH of the expression product.
[0141] The results are shown in Table 2. The results showed that the pH of the engineered bacteria SMD-pGAPZα-30 culture decreased significantly after 24 hours of culture, and then increased significantly in the middle and late stages of culture. When the pH value was between 4.0 and 5.0, the culture supernatant The antibacterial activity of the sample was high, and the anti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com