Method of synthesizing tricyclodecenyl isobutyrate spice
A technology of tricyclodecenyl isobutyrate and isobutyric acid is applied in the preparation of carboxylate, chemical instruments and methods, preparation of organic compounds, etc. The aroma is sour and other problems, so as to reduce the production cost, the process is easy to implement, and the aroma is pure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
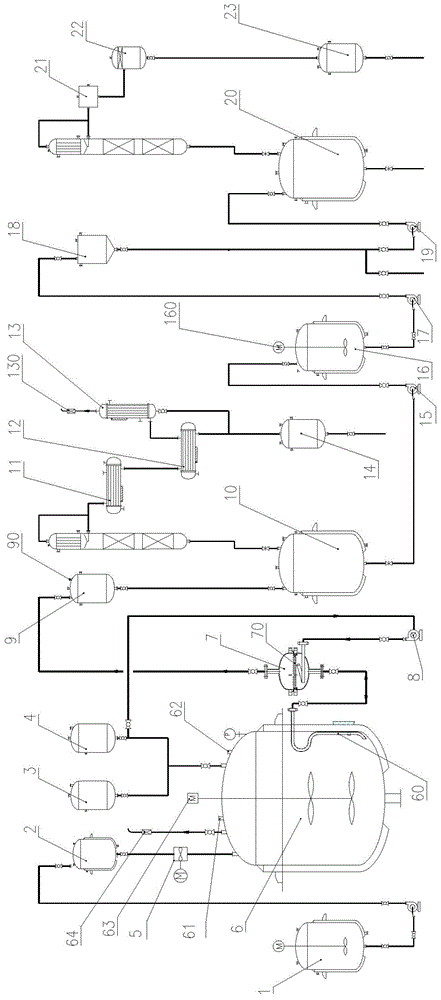
[0047] a) Pump the material in the dicyclopentadiene insulation tank 1 into the dicyclopentadiene head tank 2, add 140kg of isobutyric acid from the isobutyric acid head tank 4, and transfer from the isobutyric anhydride head tank 3 to the esterification Add 5 kg of isobutyric anhydride into the reaction kettle 6. After the feeding is completed, add 2 kg of phosphotungstic acid supported by catalyst zeolite through the hand hole 62 of the esterification reaction kettle 5, and mix evenly through the mixer 63.
[0048] b) Open the steam valve, heat up the esterification reaction kettle 6, and control the reaction temperature to 90-95°C.
[0049] c) Metering and dropping 200 kg of dicyclopentadiene in the 500L esterification reactor 6 from the dicyclopentadiene head tank 2 through the dicyclopentadiene dripping pump 5, and the dropping time is controlled at 6 hours, After the dropwise addition, keep warm and continue to react for 2 hours, then take samples for gas chromatography ...
Embodiment 2
[0056] a) Pump the material in the dicyclopentadiene insulation tank 1 into the dicyclopentadiene head tank 2, add isobutyric acid, 400kg, from the isobutyric anhydride head tank 3 to the ester Add 15 kg of isobutyric anhydride into the reaction kettle 6, after the feeding is finished, add 8 kg of phosphotungstic acid supported by catalyst zeolite from the hand hole 62 of the esterification reaction kettle 5, and start the mixer 63 to mix evenly.
[0057] b) Open the steam valve, heat up the esterification reactor 6, and control the reaction temperature to 92-95°C.
[0058] c) Metering and dropping 600 kg of dicyclopentadiene in the 1500L esterification reactor 6 from the dicyclopentadiene head tank 2 through the dicyclopentadiene dripping pump 5, and the dropping time is controlled at 12 hours, After the dropwise addition, keep warm and continue to react for 2 hours, then take samples for gas chromatography detection, and then take samples for detection at intervals of 1 hour...
Embodiment 3
[0065] a) Pump the material in the dicyclopentadiene insulation tank 1 into the dicyclopentadiene head tank 2, add isobutyric acid, 260kg, from the isobutyric anhydride head tank 3 to the ester Add 10 kg of isobutyric anhydride into the reaction kettle 6. After feeding, add 3.5 kg of phosphotungstic acid supported by catalyst zeolite from the hand hole 62 of the esterification reaction kettle 5, and start the mixer 63 to mix evenly.
[0066] b) Open the steam valve, heat up the esterification reactor 6, and control the reaction temperature to 90-92°C.
[0067] c) Metering and dropping 400 kg of dicyclopentadiene in the 1000L esterification reactor 6 from the dicyclopentadiene head tank 2 through the dicyclopentadiene dripping pump 5, and the dropping time is controlled at 10 hours, After the dropwise addition, keep warm and continue to react for 2 hours, then take samples for gas chromatography detection, and then take samples for detection at intervals of 1 hour. When the con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com