Application of hMOF (Human Males Absent on the First) and small peptide of hMOF in preparing drug for alleviating or treating human arsenic poisoning
An arsenic poisoning and drug technology, applied in the field of biomedicine, can solve the problems of abnormal DNA repair reaction, chromosomal aberration, genome instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
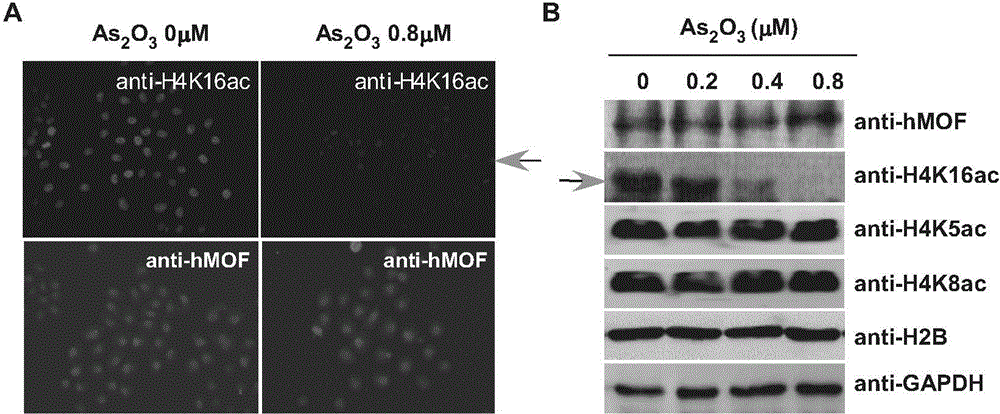
[0055] Example 1: Detection of the influence of arsenic trioxide on hMOF and its enzyme activity
[0056] 【experiment procedure】:
[0057] (1) Prepare cells: inoculate (5×10 5 per well) 293T cells (human embryonic kidney cells, purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences) and inoculated (5×10 3 per well) HeLa cells (human cervical cancer cells, purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences), and a cell slide was added to each well. 2 mL of cell culture medium was added to each well of a 6-well plate, and 0.5 mL of cell culture medium was added to each well of a 24-well plate, and cultured in a cell culture incubator (Sanyo, Japan) until the cell coverage reached 30%.
[0058] (2) Drug treatment: 293T cells were cultured until the cell coverage rate reached 30%, and four wells were selected, and each well was replaced with a new 2mL cell culture medium premixed with different arsenic concentrations, so that the final con...
Embodiment 2
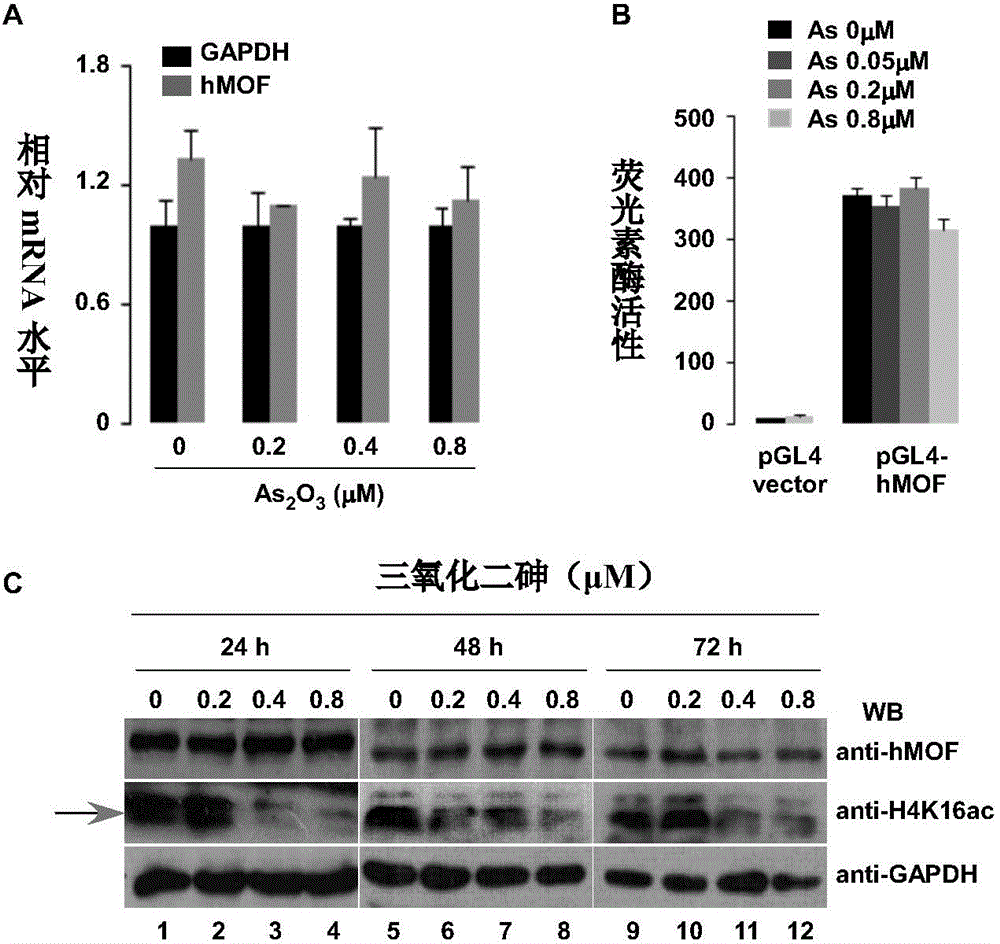
[0072] Example 2: Determination of arsenic compounds on hMOF gene expression, gene promoter region activation and hMOF protein expression at different time points
[0073] experiment procedure:
[0074] (1) RT-qPCR detection of the effect of arsenic compounds on hMOF gene expression
[0075] 293T cells (5×10 5 cells / well), after the cells were treated with 0.2-0.8 μM for 48 hours, the cell culture medium was discarded and washed once with PBS. Add 1 mL of RNAiso Plus to each well, pipette and mix with a pipette tip, incubate at room temperature for 5-10 min, add 200 μl of chloroform, shake vigorously for 15 seconds, and let stand at room temperature for 5 min. Then, the total RNA was sequentially extracted using chloroform-isopropanol-ethanol, and finally the RNA concentration and A260nm / 280nm light absorbance were measured by NanoDrop.
[0076] cDNA was obtained by reverse transcription using TAKARA kit PrimeScript Reverse Transcriptase (No.2680A), and then real-time quant...
Embodiment 3
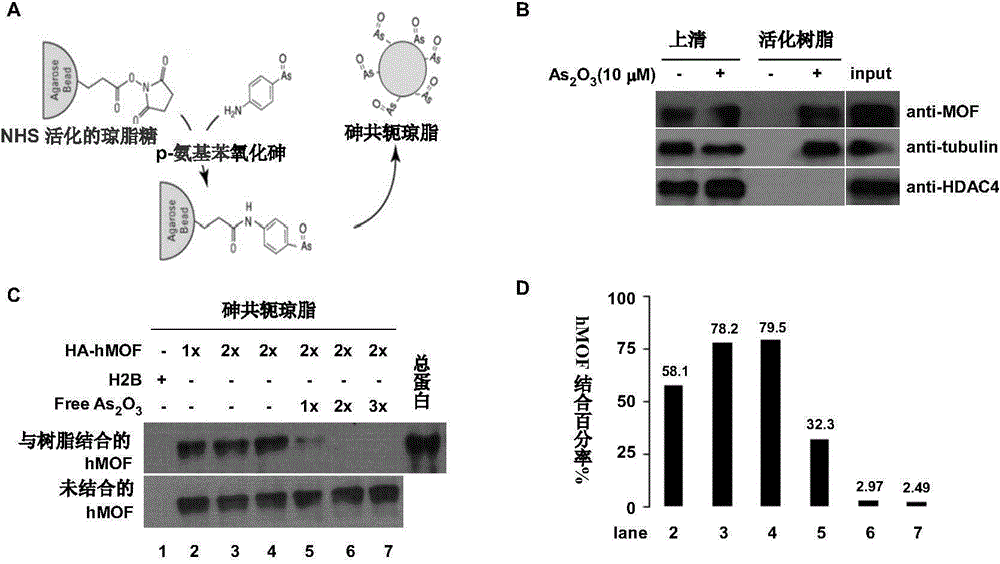
[0090] Example 3: Direct binding of arsenic compounds to hMOF
[0091] experiment procedure:
[0092] (1) Preparation of arsenic-conjugated resin
[0093] After 50 μl of NHS-activated agarose was equilibrated with coupling buffer (0.1 M phosphate, 0.15 M sodium chloride), the coupling agent PAPAO (10 μmol) was added. After 4 hours on ice, it was further eluted with coupling buffer. The remaining epoxy active sites were blocked with 1 M ethanolamine for 20 min at room temperature. Finally, the agarose gel was eluted with coupling buffer again and stored at 4°C for future use (protected from light).
[0094] (2) Affinity purification of arsenic-conjugated resin
[0095] 20 μL of prepared arsenic-conjugated resin was reacted with 200 μL of whole cell extract at 4° C. for 12 hours. Eluted three times with PBS the next day. After centrifugation, 4xSDS loading buffer (Beijing Dingguo Biotechnology Co., Ltd. Cat No-WB-0081) was added. Proteins bound to the arsenic-conjugated r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com