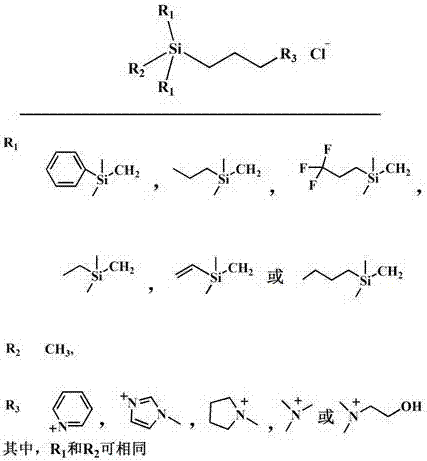
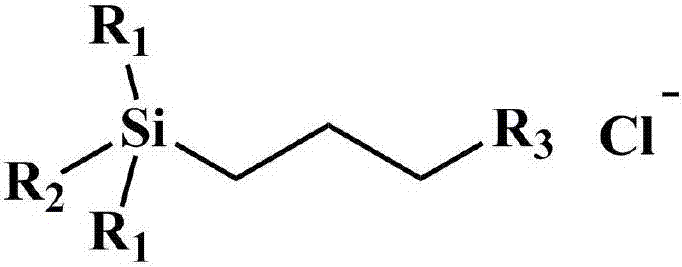
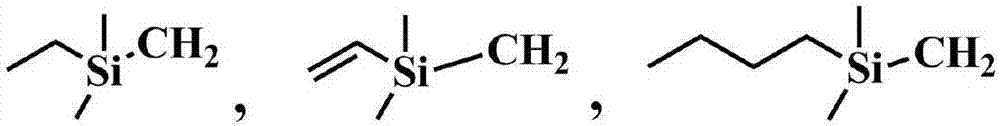A kind of hydrolysis-resistant si-c-si type cationic silicone surfactant
A surfactant and cationic technology, which is applied in the preparation of surfactants and in the field of hydrolysis-resistant Si-C-Si cationic silicone surfactants, can solve problems that are not suitable for industrial production and affect the activity of surfactants. Achieve the effects of feasible preparation method, excellent hydrolysis resistance and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] N 2 Under protection, in a 250 ml four-neck flask, add 8.6 g of chloropropylmethyldichlorosilane to 50 ml of anhydrous tetrahydrofuran, cool to 0 °C, add 8 g of trimethylsilylmethylmagnesium chloride dropwise, and let it rise naturally to room temperature and stirring was continued for 4 hours. After the reaction is completed, the reaction is terminated with anhydrous methanol, separated by filtration, extracted and separated, and concentrated to obtain chloropropyltrisilazane.
[0026] In a 100ml three-necked flask equipped with a condenser, add 5.0 g chloropropyl trisilane, 4.0 g N-methylimidazole, 10 ml isopropanol, N 2 React at 85°C for 36 hours under protection, and cool to room temperature naturally after the reaction. Pour into 30 ml of ethyl acetate to precipitate the crude product, pour off the upper layer solution, and then add 2 ml of anhydrous methanol to dissolve the crude product. Then the dissolved product was added to 30 ml of ethyl acetate to precipi...
Embodiment 2
[0028] N 2 Under protection, in a 250 ml four-necked flask, 8.6 g of chloropropylmethyldichlorosilane was added to 50 ml of anhydrous tetrahydrofuran, cooled to 0 ° C, and 12 g of phenyldimethylsilylmagnesium chloride Grignard reagent was added dropwise, Naturally rose to room temperature and continued to stir for 5 hours. After the reaction is completed, the reaction is terminated with anhydrous methanol, separated by filtration, extracted and separated, and concentrated to obtain chloropropyltrisilazane.
[0029] In a 100ml three-necked flask equipped with a condenser, add 6.0 g of phenylchloropropyl trisilane, 4.0 g of N-methylimidazole, 10 ml of isopropanol, N 2 React at 85°C for 36 hours under protection, and cool to room temperature naturally after the reaction. Separation with n-hexane, and then remove the n-hexane to obtain a solid at 0.1 MPa, dry at 40°C for 1 day, and obtain a phenyl-containing Si-C-Si type silicone surfactant after cooling. The yield was 89%. Ke...
Embodiment 3
[0031] N 2 Under protection, in a 250 ml four-necked flask, add 9.1 g of chloropropylmethyldimethoxysilane to 50 ml of anhydrous tetrahydrofuran, add 8 g of trimethylsilylmagnesium chloride Grignard reagent dropwise, and heat up after the addition Slightly reflux and stirring was continued for 4 hours. After the reaction is completed, the reaction is terminated with anhydrous methanol, separated by filtration, extracted and separated, and concentrated to obtain chloropropyltrisilazane.
[0032] In a 100ml three-necked flask equipped with a condenser, add 5.0 g chloropropyl trisilane, 4.0 g N-methylimidazole, 10 ml isopropanol, N 2 React at 85°C for 36 hours under protection, and cool to room temperature naturally after the reaction. Pour into 30 ml of ethyl acetate to precipitate the crude product, pour off the upper layer solution, and then add 2 ml of anhydrous methanol to dissolve the crude product. Then the dissolved product was added to 30 ml of ethyl acetate to precip...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com