Omeprazole sodium semihydrate and preparation method thereof
A technology of omeprazole sodium and hemihydrate, which is applied in the field of chemical engineering and pharmaceutical crystallization, can solve the problems of long time, 10-24 hours, low thermal decomposition temperature, poor stability and the like, and achieves difficult coalescence and molar yield. High and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
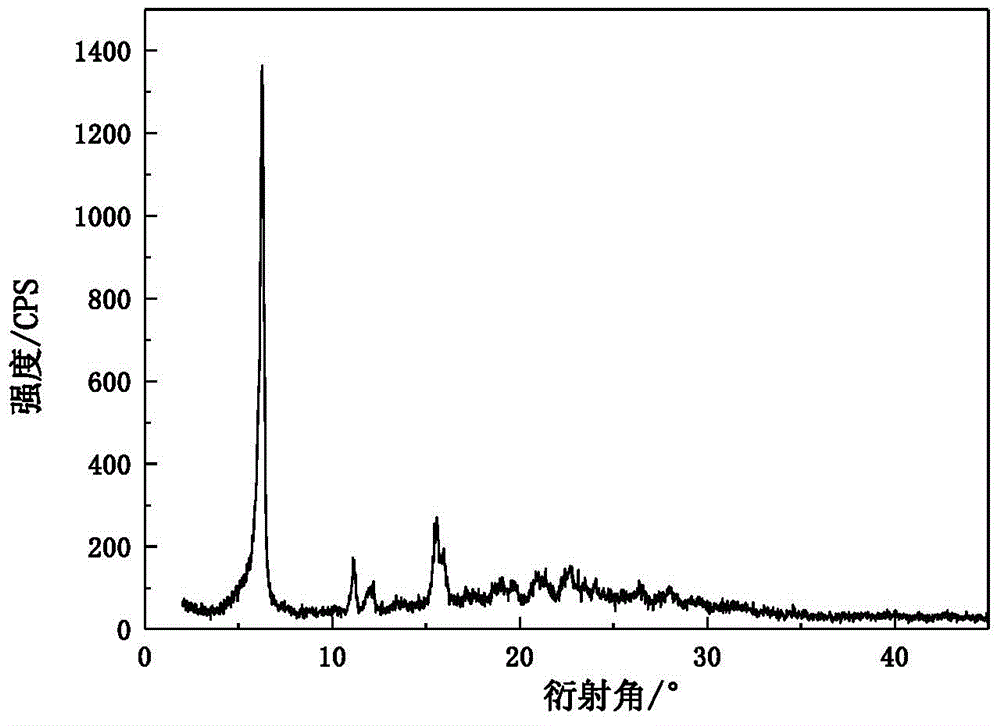
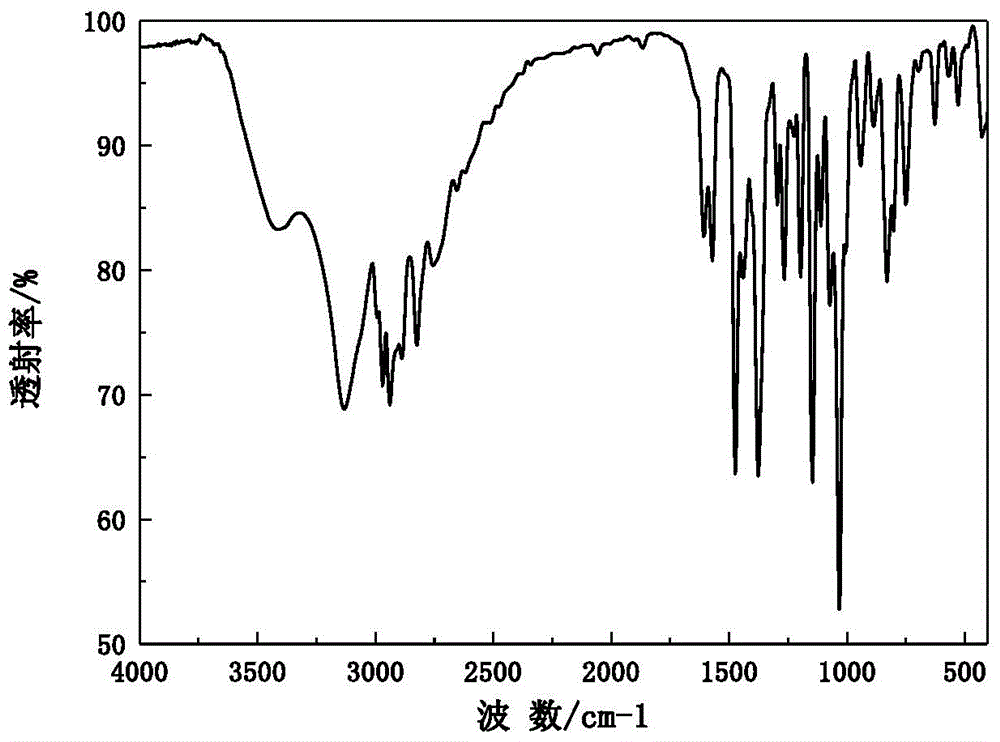
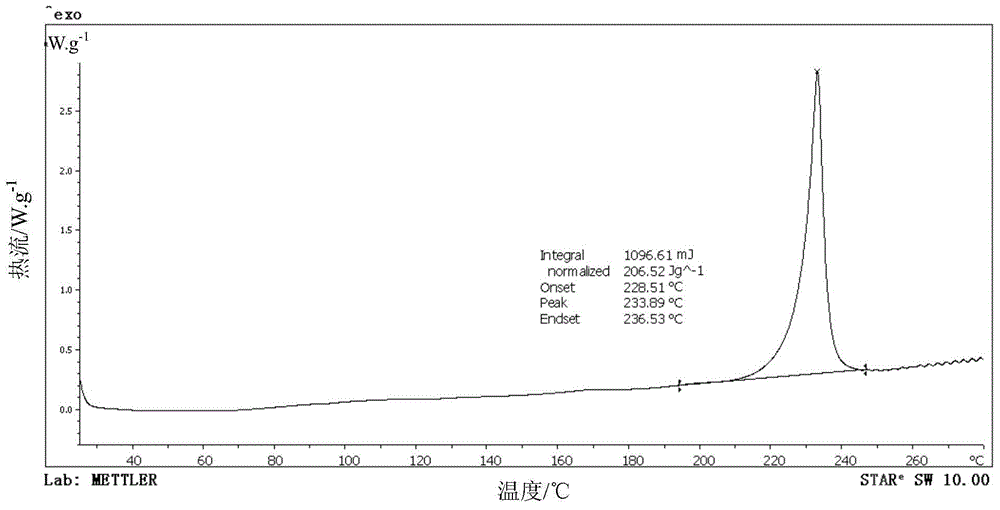
[0038] Add 0.45g omeprazole sodium monohydrate, 20mL ethyl acetate and 10mL sec-butanol to the crystallizer, stir for 2 hours at 35°C and a magnetic sub-rotation speed of 200r / min, filter and dry to obtain omeprazole Sodium hemihydrate 0.42g, molar yield 95.6%, HPLC content 99.8%. X-ray powder diffraction pattern of the product and attached figure 1 Consistent, solid-state Fourier transform infrared spectroscopy and attached figure 2 Consistently, the DSC decomposition temperature is 227.5°C. The main particle size of the crystal is 92 μm, no coalescence, and the Carr index is 14.3%, indicating that it has good fluidity. It is still a white crystal after being placed at a high temperature of 60°C for ten days, and the X-ray diffraction pattern does not change significantly. The result of the thermal stability test at 60°C shows that the weight change rate is 2.4% in 10 days, and the thermal stability is good. Under the conditions of 40°C and 75% relative humidity for 3 day...
Embodiment 2
[0040] Add 2.40g omeprazole sodium monohydrate and 20mL methyl isobutyl ketone to the crystallizer, stir for 7 hours at 60°C and a mechanical stirring speed of 300r / min, filter, dry at 35°C and a vacuum of 0.05MPa to obtain Omeprazole sodium hemihydrate 2.27g, molar yield 96.8%, HPLC content 100.2%. The X-ray powder diffraction pattern of the product has characteristic peaks at diffraction angles 2θ of 6.34, 11.18, 12.26, 15.58, 16.02, 17.06, 19.12, 21.04, 22.66, 23.52, 24.10, 26.44 and 28.04 degrees. Solid Fourier transform infrared The spectra are at wavenumbers 3414.5, 3132.1, 2969.9, 2939.8, 2846.9, 2756.5, 1607.7, 1571.9, 1474.5, 1441.5, 1377.3, 1266.0, 1147.8, 1112.3, 1072.8, 1032.9, 901.3, 83, 83 -1 There are characteristic peaks, and the DSC decomposition temperature is 229.2°C. The main particle size of the crystal is 95 μm, no coalescence, and the Carr index is 13.9%, indicating that it has good fluidity. It is still a white crystal after being placed at a high tem...
Embodiment 3
[0042] Add 3.00 g of omeprazole sodium hydrate (water content 7.02%), 10 mL of tetrahydrofuran, 10 mL of formamide and 10 mL of methyl ethyl ketone into the crystallizer, and stir for 9 hours at 25° C. with a mechanical stirring speed of 600 r / min. Filter and dry at 30°C under a vacuum of 0.1 MPa to obtain 2.78 g of omeprazole sodium hemihydrate with a molar yield of 97.4% and an HPLC content of 100.0%. The X-ray powder diffraction pattern of the product has characteristic peaks at diffraction angles 2θ of 6.28, 11.16, 12.16, 15.62, 16.02, 17.02, 19.10, 21.00, 22.60, 23.44, 24.16, 26.42 and 28.02 degrees. Solid Fourier transform infrared The spectra are at wavenumbers 3413.0, 3132.3, 2968.7, 2938.1, 2846.8, 2754.6, 1605.4, 1572.1, 1474.6, 1441.5, 1375.8, 1266.2, 1146.8, 1111.0, 1074.2, 10362.9, 712.8, 83.8 cm -1 There are characteristic peaks, and the DSC decomposition temperature is 229.5°C. The main particle size of the crystal is 100μm, no coalescence, and the Carr index i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal degradation temperature | aaaaa | aaaaa |
| Carr index | aaaaa | aaaaa |
| Carr index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com