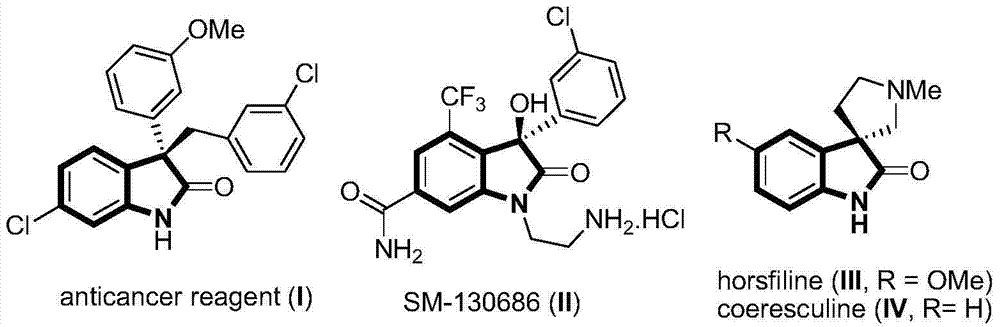
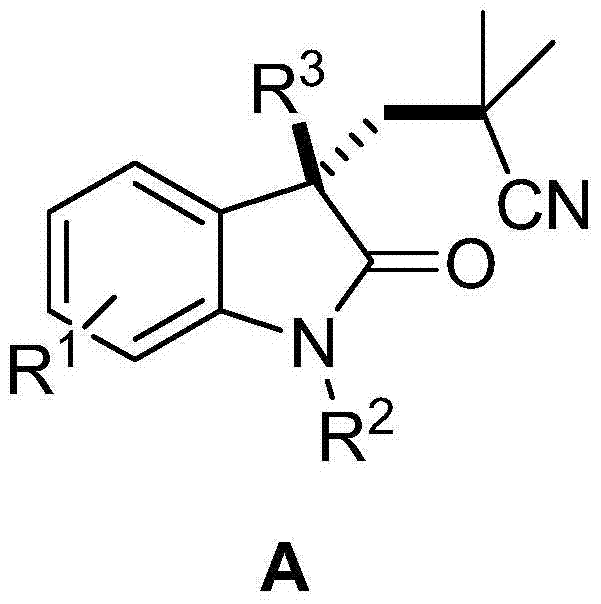
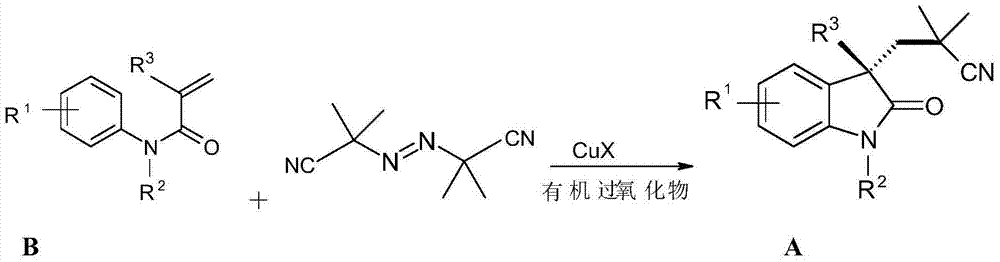
3-(2,2-dimethyl) propionitrile-3-alkyl (aryl) indolone and preparation method thereof
A technology of dimethyl and propylcyano, which is applied in the field of simple synthesis, drug construction skeleton 3-propylcyano-3-alkylindolinone and its low cost, and can solve the problems of inability to synthesize
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0031] The preparation of raw material B, taking the preparation of N-methyl-N-phenyl 2-methacrylamide as an example
[0032]
[0033] N-Methyl-N-phenyl 2-methacrylamide
[0034] Add 2-methacrylic acid (15mmol) and dry dichloromethane 20mL in a 100mL round-bottomed flask, then stir the mixture for 5min in ice bath, add 3-5 drops of DMF (N,N-dimethylformamide ), then slowly add equivalent oxalyl chloride (16.5mmol) dissolved in 5-10mL of dry dichloromethane, and then react at 0°C for 1h (opening the reaction system is conducive to the volatilization of hydrogen chloride gas generated). Then, under ice-bath conditions, triethylamine (30mmol, 2eq) dissolved in a small amount of dry dichloromethane was added, followed by aniline (10mmol, also dissolved in a small amount of dry methylene chloride, reacted in a closed system at room temperature for 1-2h. The mixture was washed with saturated brine, filtered, and the filtrate was distilled off to obtain a crude product. Using eth...
Embodiment 1
[0055] Preparation of target product A, taking the preparation of 3-(2,2-dimethyl)propylcyano-1,3-dimethylindol-2-one as an example
[0056]
[0057] Add N-methyl-N-phenyl-β-methyl-acrylamide (0.5mmol.0.052g), azobisisobutylcyanide AIBN (1.0mmol, 0.164g) and copper iodide successively in a round bottom flask (0.002mmol, 0.004g), acetonitrile (2mL), and finally add di-tert-butyl peroxide DTBP (1.0mmol, 0.146g), react at 80°C, after the reaction (usually 3-5 hours) , ethyl acetate was added, and then washed with brine, and the aqueous phase was extracted with ethyl acetate. The organic phase was collected, dried, concentrated, and separated by column chromatography to obtain the product 3-(2,2-dimethyl)propylcyano-1,3-dimethylindol-2-one, a colorless solid, melting point : 89-90°C, yield 75%.
[0058] Structure Characterization: 1 H NMR (400MHz, CDCl 3 )δ7.40-7.25 (m, 2H), 7.09 (t, J = 6.0Hz, 1H), 6.90 (d, J = 6.4Hz, 2H), 3.23 (s, 3H), 2.32 (d, J = 11.6 Hz,1H),2.16(d,J=1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com