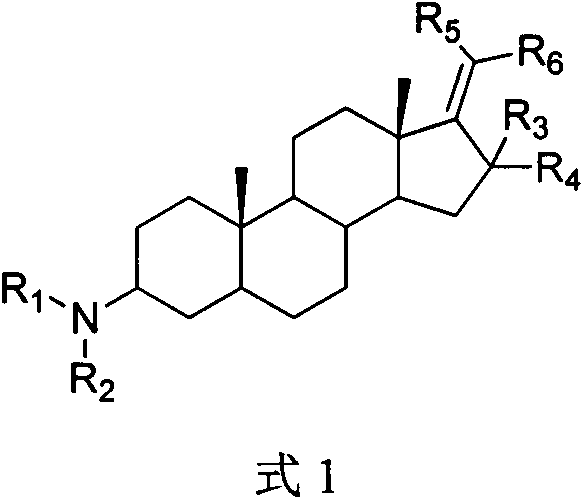
Pregnane alkaloid derivative with effect of resisting breast cancer metastasis and medical application of pregnane alkaloid derivative
A technology of alkaloid derivatives and pregnane, which is applied in the field of medicinal chemistry research and can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
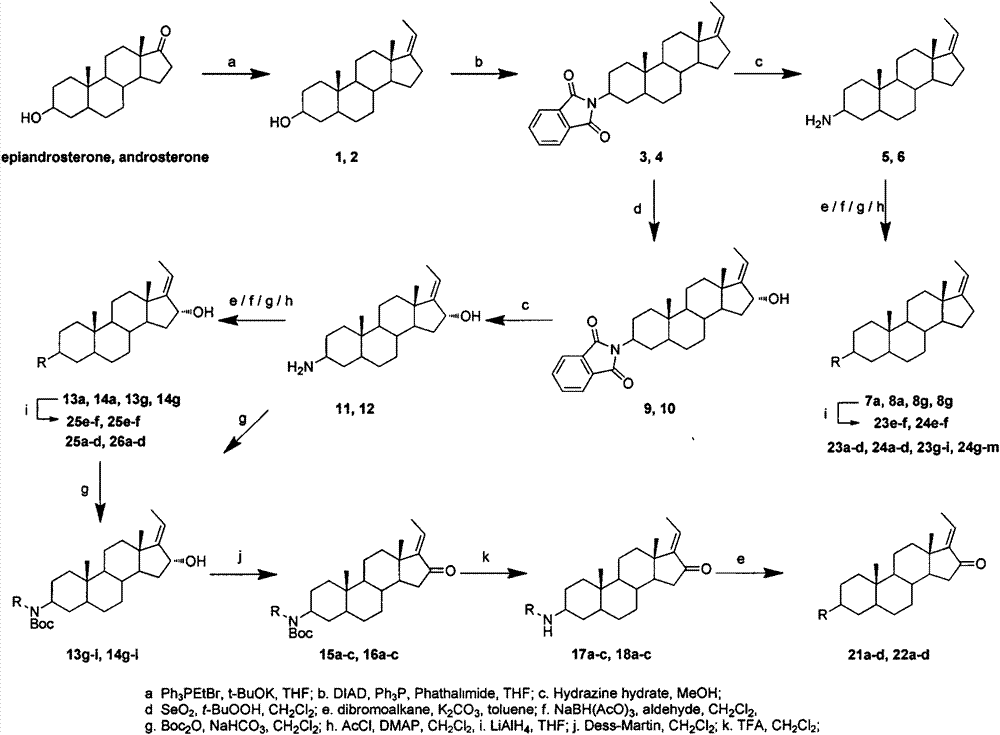
[0042] The synthetic method of compound 1 and 2
[0043] Weigh 4 equivalents of potassium tert-butoxide and 4 equivalents of ethyltriphenylphosphine bromide into a reaction flask, add an appropriate amount of anhydrous THF, and stir at room temperature for 1 hour. Add 1 equivalent of epiandrosterone or androsterone to the reaction solution, reflux under nitrogen protection, and TLC monitors that the reaction is complete. Add saturated ammonium chloride aqueous solution to terminate the reaction, extract with dichloromethane, wash with water three times, combine the organic phases, dry over anhydrous magnesium sulfate, filter, and concentrate to obtain a crude product. The crude product was recrystallized from dichloromethane-methanol to obtain pure product.
[0044] Synthesis of (Z)-3β-hydroxypregna-17(20)-ene(1)
[0045]
[0046] Using epiandrosterone as a raw material, it was prepared according to the above method, and the crude product was recrystallized from methanol ...
Embodiment 2
[0051] The synthetic method of compound 3 and 4
[0052] Weigh 1.13 equivalents of triphenylphosphine, 1.1 equivalents of phthalimide and 1 equivalent of compound 1 or 2 in a reaction flask, add an appropriate amount of anhydrous tetrahydrofuran and stir for 1 hour under an ice bath. Add 2 equivalents of diisopropyl azodicarboxylate to the reaction solution, stir at room temperature under seal, and monitor by TLC until the reaction is complete. Add water to quench the reaction, concentrate under reduced pressure to remove THF, extract with dichloromethane, wash with water three times, combine organic phases, dry over anhydrous magnesium sulfate, filter, and concentrate to obtain a crude product. The crude product was recrystallized to obtain pure product.
[0053] Synthesis of (Z)-3β-phthalimidopregna-17(20)-ene(3)
[0054]
[0055] Using 9.66 g of compound 2 as a raw material, it was prepared according to the above method, and the crude product was recrystallized from a ...
Embodiment 3
[0060] The synthetic method of compound 5,6,11 and 12
[0061] Weigh 1 equivalent of raw materials into a reaction flask, add an appropriate amount of methanol and 40 equivalents of 80% hydrazine hydrate to reflux. TLC monitored the end of the reaction. Cool to room temperature, add an appropriate amount of sodium hydroxide aqueous solution (4-6mol / L) until the solution turns white and turbid. Filtrate with suction, wash with water, and dry to obtain a crude product, which is purified by HW-40 gel column chromatography to obtain a pure product.
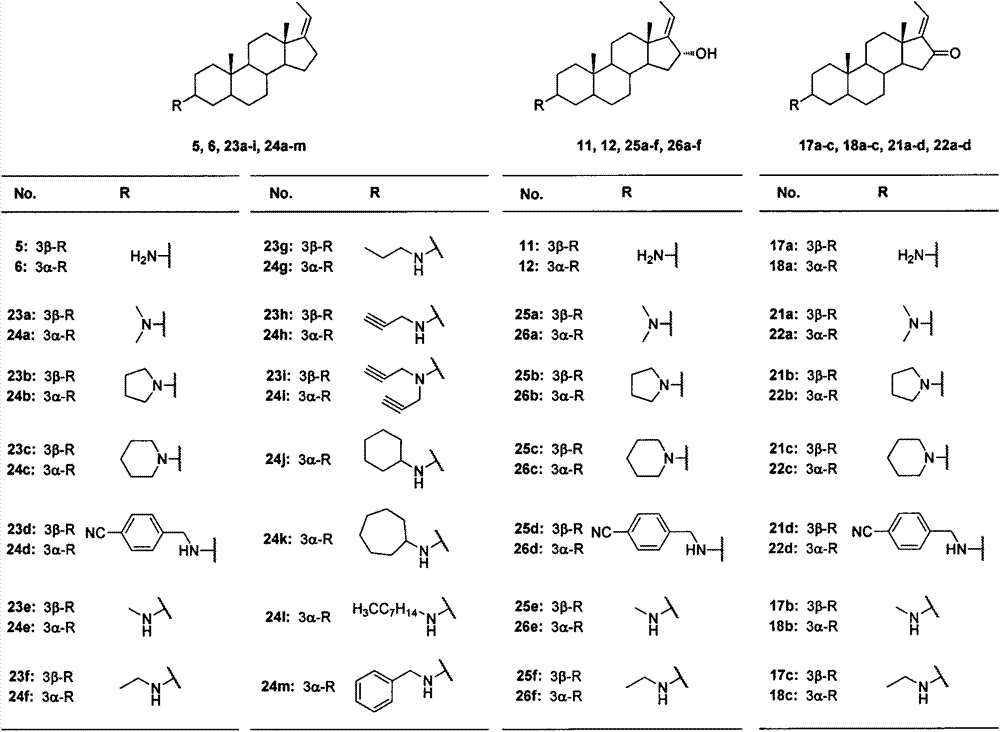
[0062] Synthesis of (Z)-3β-aminopregna-17(20)-ene(5)
[0063]
[0064] Using compound 3 as raw material, it was prepared according to the above method, and the crude product was purified by HW-40 gel column chromatography (DCM:MeOH=2:1, V / V) to obtain a white solid with a yield of 68.3%. 1 H NMR (400MHz, CDCl 3 )δ5.10(qt, J=2.0, 6.8Hz, 1H), 2.67(br s, 1H), 1.64(dt, J=2.0, 6.8Hz, 3H), 0.86(s, 3H), 0.79(s, 3H). 13 C NMR (100MHz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com