Glucocorticoid aerosol inhalation suspension and preparation method thereof
A glucocorticoid, atomized inhalation technology, applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., to achieve uniform appearance, high yield, and the effect of ensuring the health of production personnel
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention provides a kind of preparation method of glucocorticoid atomized inhalation suspension, is characterized in that, comprises:
[0043] Step 1: preparing a mixed solution: dispersing the glucocorticoid drug in an aqueous solution to obtain a mixed solution containing a glucocorticoid-insoluble drug;
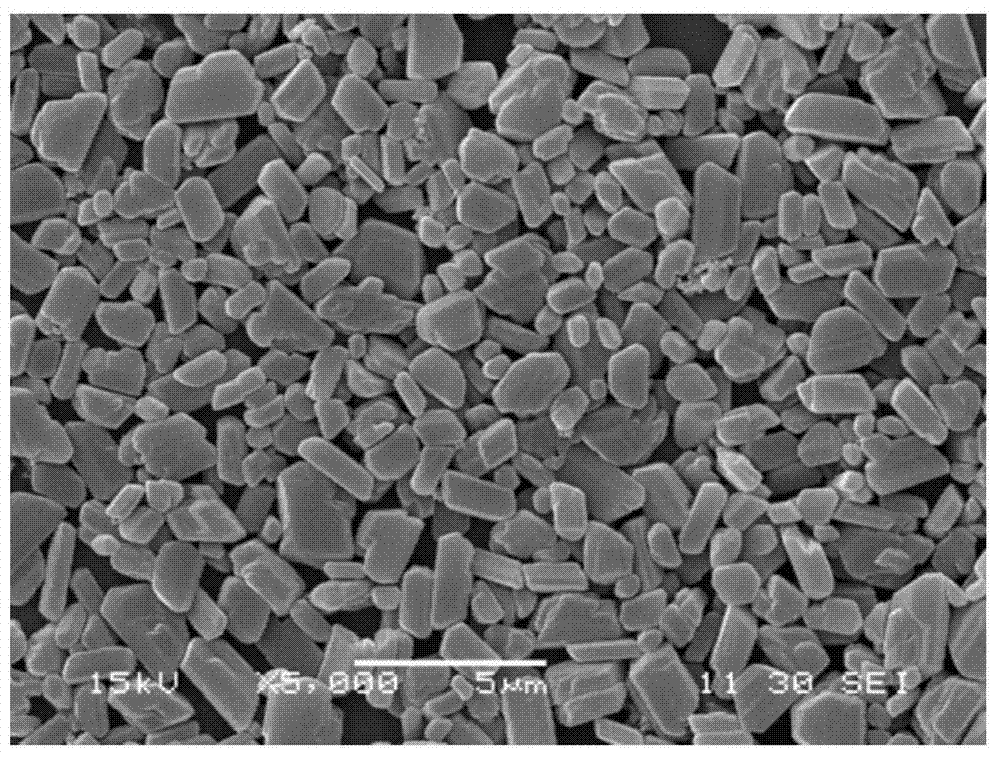
[0044] Step 2: high-pressure homogenization: homogenize the mixed solution containing the glucocorticoid-insoluble drug through a high-pressure homogenizer until the particle size distribution of the glucocorticoid-insoluble drug is d(v,0.5)=1-3um , to obtain the glucocorticoid atomized inhalation suspension.
[0045]In a preferred embodiment of the present invention, the aqueous solution is water for injection, and the glucocorticoid is preferably a medically useful glucocorticoid, more preferably including fluticasone propionate, budesonide, dexamethasone, Prednisone acetate, methylprednisolone, prednisolone, hydrocortisone, beclomethasone dipropiona...
Embodiment 1
[0057] 1. A single component of a glucocorticoid atomized inhalation suspension and its dosage
[0058]
[0059] 2. The preparation method of the above-mentioned glucocorticoid atomized inhalation suspension
[0060] Step 1: Prepare a mixed solution: disperse fluticasone propionate in an aqueous solution containing Tween and Span by magnetic stirring to obtain a mixed solution containing fluticasone propionate;
[0061] Step 2: High-pressure homogenization: Homogenize the fluticasone propionate mixture through a high-pressure homogenizer until the particle size distribution of the raw material drug is d(v,0.5)=1-3um to obtain a suspension, wherein the high-pressure The homogenization pressure is 1000bar;
[0062] Step 3: Potting: After high-pressure homogenization, the suspension is filled into 2ml ampoules;
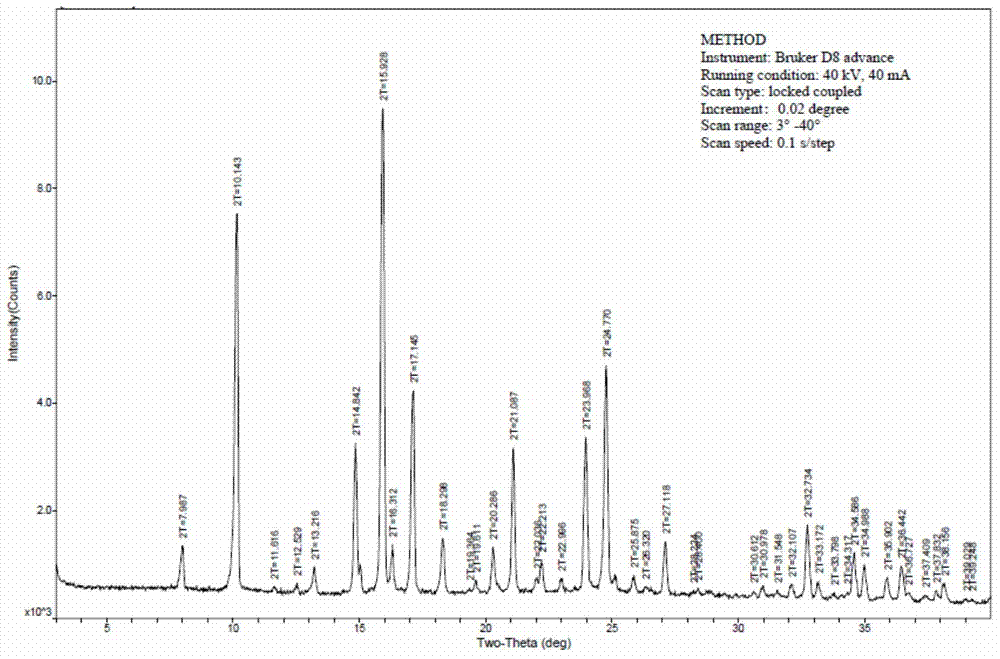
[0063] Step 4: Sterilization: Sterilize at 121° C. for 15 minutes to obtain the glucocorticoid aerosol inhalation solution suspension.
[0064] 3. Detection
[00...
Embodiment 2
[0079] 1. A single component of a glucocorticoid atomized inhalation suspension and its dosage
[0080] single component
Dosing
Beclomethasone dipropionate
0.8mg
0.2mg
glucose
48.0mg
Stearyl polyoxyethylene (2) ether
0.45mg
Sodium dihydrogen phosphate dihydrate
18.8mg
[0081] Disodium phosphate
3.5mg
Water for Injection
Add water to 2mL
[0082] 2. The preparation method of the above-mentioned glucocorticoid atomized inhalation suspension
[0083] Step 1: preparing a mixed solution: dispersing budesonide in an aqueous solution containing Tween by magnetic stirring to obtain a mixed solution containing budesonide;
[0084] Step 2: High-pressure homogenization: Homogenize the beclomethasone dipropionate mixture through a high-pressure homogenizer until the particle size distribution of the raw material drug is d(v,0.5)=1-3um to obtain a suspension, where...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com