Recombinant anti-insect protein and its preparation method and application
A kind of anti-insect protein and protein technology, applied in the field of recombinant anti-insect protein and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
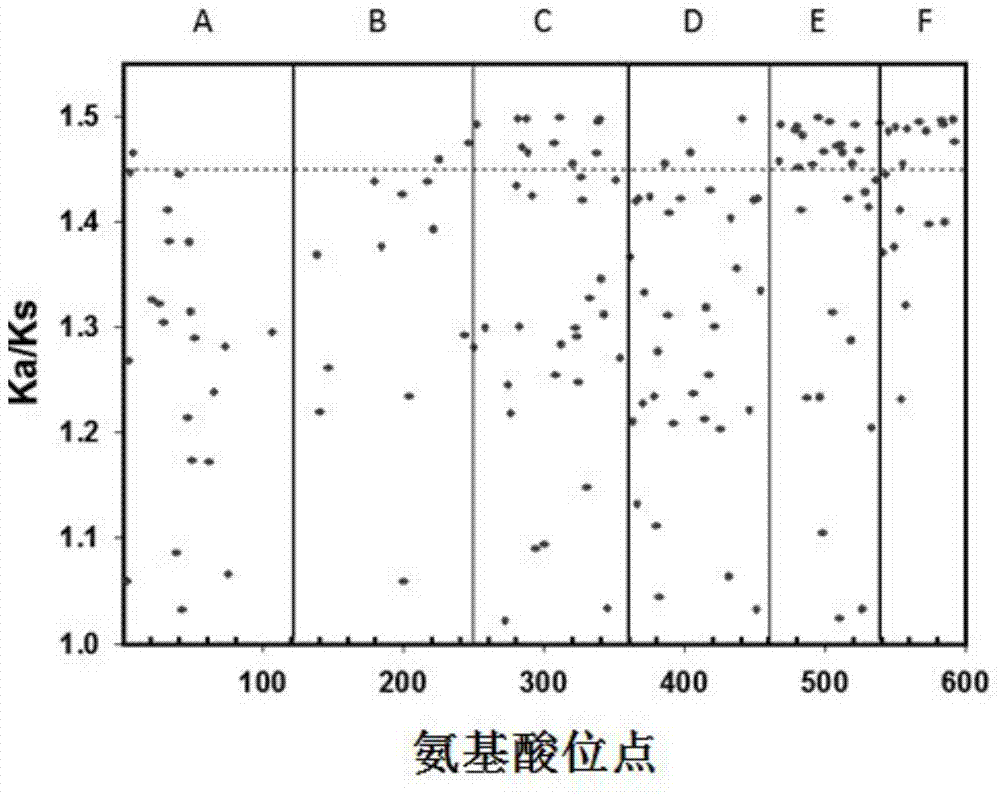
[0061] Example 1 Determination of the modified region of the Cry protein family
[0062](1) Obtain the amino acid sequences of some members of the Cry protein family from the NCBI database. The 24 selected Cry proteins are Cry1A105 protein, Cry1Aa1 protein, Cry1Ab1 protein, Cry1Ab5 protein, Cry1Ab14 protein, Cry1Ac1 protein, Cry1Ah1 protein, Cry1Ba1 protein, Cry1Bb1 protein protein, Cry1Be1 protein, Cry1Bf1 protein, Cry1Fa1 protein, Cry1Gc protein, Cry1Ia1 protein, Cry1If1 protein, Cry1Ja1 protein, Cry1Jb1 protein, Cry1Jc1 protein, Cry2Ab1 protein, Cry2Ac1 protein, Cry2Ae1 protein, Cry9Aa1 protein, Cry9Ca1 protein, Cry9Ec1 protein.
[0063] (2) Using the pMAL algorithm and perl programming language, the evolution rate analysis method is used to conduct statistical analysis on the above 24 Cry proteins, predict the highly evolutionary activity regions of the above 24 Cry protein amino acid sequences in the molecular evolution process, and determine the evolution rate The fastes...
Embodiment 2
[0066] Example 2 Constructing an insect-resistant gene bank
[0067] (1) Select the genes with anti-lepidoptera activity in the NCBI database to establish a data set, and perform local similarity comparison by BLAST, that is, BLAST comparison.
[0068] Cry genes from different subfamilies were selected according to the difference of quaternary nucleotide homology:
[0069] Cry1Ab1 gene, Cry1Ab5 gene, Cry1Ab14 gene: nucleotide homology greater than 95%;
[0070] Cry1Ab gene, Cry1Ac gene, Cry1Ah gene: nucleotide homology between 78% and 95%;
[0071] Cry1A gene, Cry1B gene, Cry1F gene, Cry1G gene, Cry1I gene, Cry1J gene: nucleotide homology between 45% and 78%;
[0072] Cry1 gene, Cry2 gene, Cry9 gene: the nucleotide homology is less than 45%.
[0073] (2) Since the selected Cry genes are closely related genes, the maximum parsimony method (Maximum parsimony, MP method) was used to establish a phylogenetic tree: firstly, the Clustalx2.0 software was used to perform multiple c...
Embodiment 3
[0083] Example 3 Construction of recombinant Cry protein
[0084] The plasmid pET28 and the 64 genes constructed in Example 2 were digested with NcoI endonuclease and BamHI endonuclease respectively; the 64 genes digested with double enzymes were respectively connected to the plasmid pET28 to complete the construction of 64 vectors; The 64 constructed vectors were verified step by step by PCR, double-enzyme hydrolysis and sequencing; the verified correct vectors were transferred into the expression host E.coliBL21(DE3)plysS for induced expression, and 64 recombinant Cry proteins were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com