A meso-position arylamino monosubstituted porphyrin derivative and its preparation method
A derivative and mono-substitution technology, which is applied in the field of meso-position arylamino mono-substituted porphyrin derivatives and its preparation, can solve the problems of less research on compound synthesis, greater difficulty, and more steps in porphyrin synthesis, achieving less pollution, The effect of good reaction selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
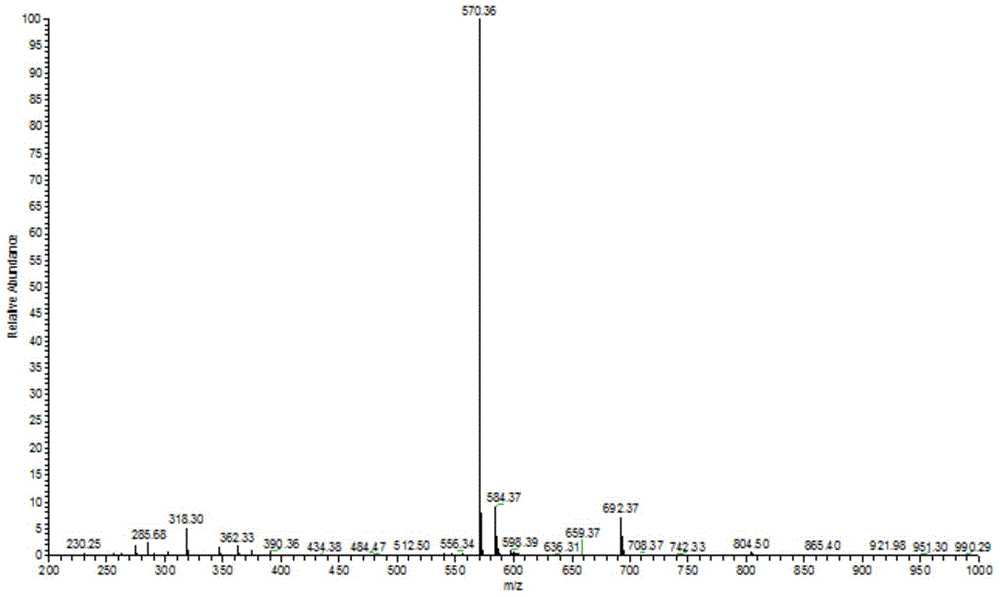
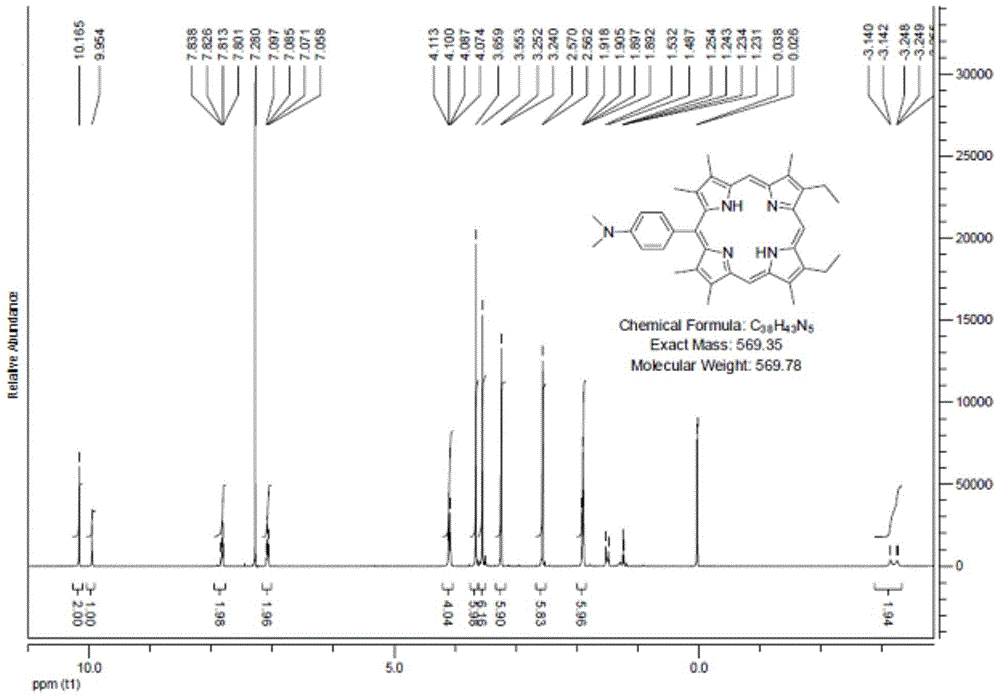
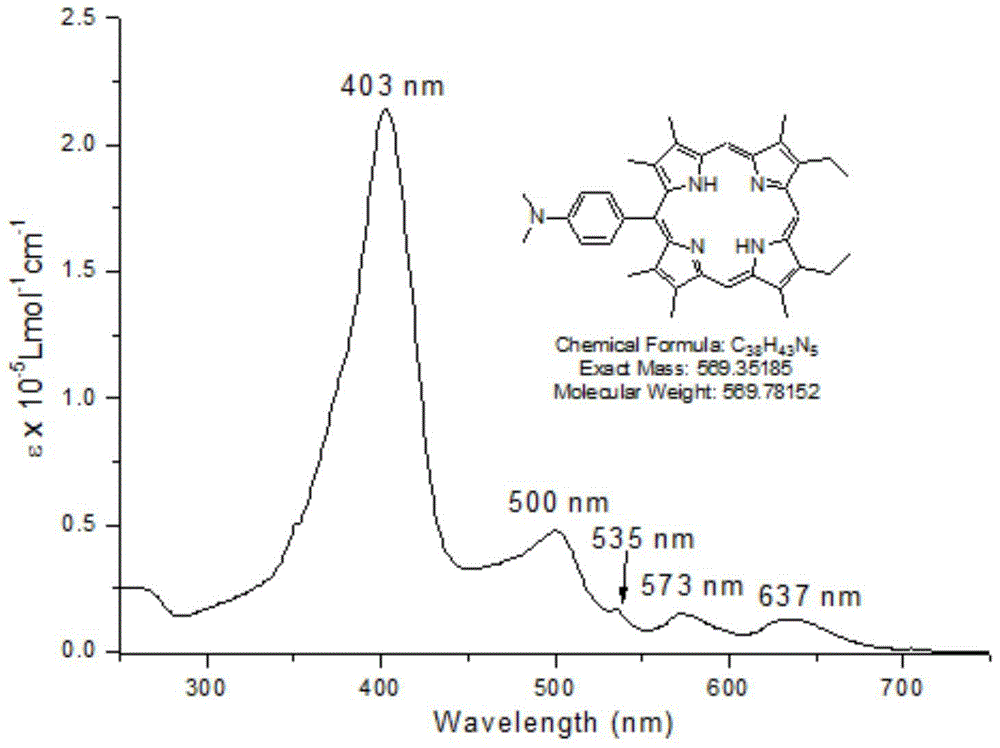
[0026] 4-N,N-Dimethylaminobenzaldehyde (1mmol) and dibromo-1,19-dideoxy-3,8,12,17-tetraethyl-2,7,13,18-tetramethyl Add biladien-a, c (1mmol) into a 250mL three-necked flask, and add ethanol (100mL) to dissolve. The solution was heated to reflux (78-82°C) under argon protection. p-Toluenesulfonic acid (2.5 g) was dissolved in ethanol (10 mL) and added slowly to the reaction mixture over 18 hours. The reaction mixture was then continued for up to 48 hours. After the reactant was cooled to room temperature, the solvent was removed under reduced pressure. The residue was dissolved in 200 mL DCM and washed with saturated NaHCO 3 The solution and distilled water were washed twice, and the aqueous layer was removed. The solvent was evaporated under reduced pressure for chromatographic separation, and the product 5-(4-N,N-dimethyl)phenyl-13,17-diethyl-2,3,7,8 was obtained after recrystallization from methanol and chloroform , 12,18-Hexamethylporphyrin (1a). Yield: 42.1%. Meltin...
Embodiment 2
[0028] Synthesis of 5-(4-carbazolyl)phenyl-13,17-diethyl-2,3,7,8,12,18-hexamethylporphyrin (1b): 4-carbazolylbenzaldehyde (1mmol) and dibromo-1,19-dideoxy-3,8,12,17-tetraethyl-2,7,13,18-tetramethylbiladien-a,c (1mmol) into a 250mL three-necked flask, And add ethanol (100mL) to dissolve, other methods are the same as Example 1, productive rate: 29.4%. Melting point: >250℃; Esi-MS: calcd for C 48 h 46 N 5 692.38, found: 692.38 (M+H + )( Figure 4 ); 1 H-NMR (600MHz, CDCl 3 )δ10.20(2H, s), 10.00(1H, s), 8.31(4H, m), 7.95(2H, d), 7.77(2H, d), 7.64(2H, m), 7.45(2H, d ), 4.10(4H, q), 3.68(6H, s), 3.61(6H, s), 2.71(6H, s), 1.91(6H, t), -3.12(1H, s), -3.26(1H, s)( Figure 5 ; UV-vis: 403nm, 503nm, 536nm, 570nm, 625nm ( Figure 6 ).
Embodiment 3
[0030] 5-(4-(3,6-di-tert-butyl)carbazolyl)phenyl-13,17-diethyl-2,3,7,8,12,18-hexamethylporphyrin (1c) Synthesis of: 4-(3,6-di-tert-butylcarbazolyl)benzaldehyde (1mmol) and dibromo-1,19-dideoxy-3,8,12,17-tetraethyl-2,7, 13,18-Tetramethyl biladien-a,c (1 mmol) was added into a 250 mL three-neck flask, and ethanol (100 mL) was added for dissolution. The other methods were the same as in Example 1, and the yield: 32.5%. Melting point: >250℃; Esi-MS: calcd forC 56 h 62 N 5 804.5005, found: 804.5026 (M+H + )( Figure 7 ); 1 H-NMR (600MHz, CDCl 3 )δ10.20(2H, s), 9.99(1H, s), 8.32(2H, s), 8.19(2H, d), 7.89(2H, d), 7.71(4H, d), 4.10(4H, q ), 3.67(6H, s), 3.59(6H, s), 2.66(6H, s), 1.91(6H, t), 1.60(18H, s), -3.14(1H, s), -3.26(1H, s)( Figure 8 ); UV-vis: 403nm, 501nm, 536nm, 570nm, 622nm ( Figure 9 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com