Piribedil sustained-release tablet and preparation method thereof
A technology of piribedil and sustained-release tablets, which is applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, and medical preparations containing active ingredients, etc., which can solve the problems of difficult drug release and poor correlation between in vivo and in vitro, etc. Achieve the effects of simple process, improved drug efficacy and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
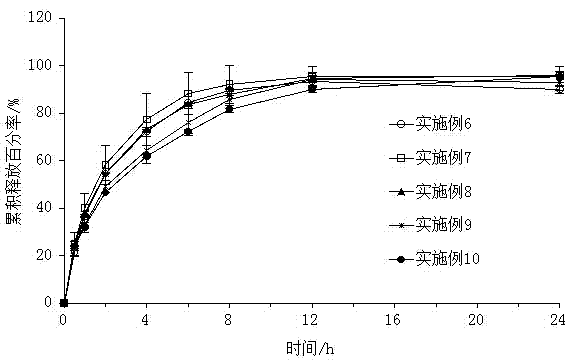
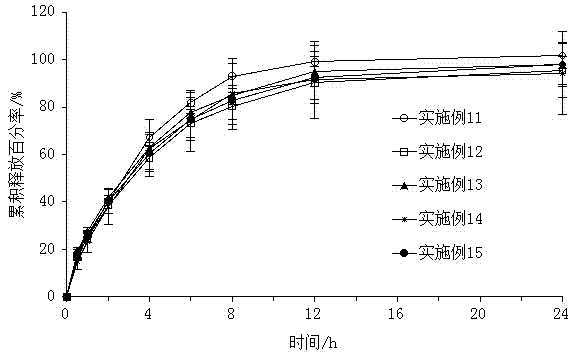
Examples
Embodiment 1
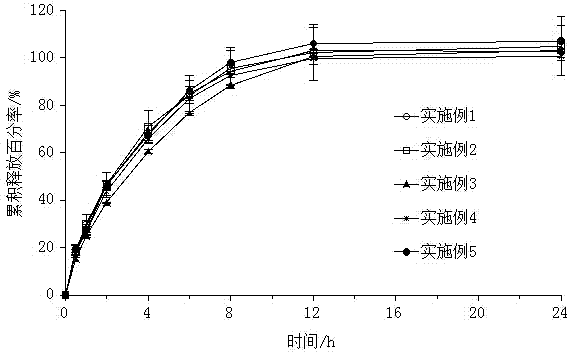
[0032] Embodiment 1: Preparation of piribedil sustained-release tablets
[0033] Prepare 25g piribedil sustained-release tablets, first take by weighing piribedil that accounts for 38.5% of the quality of piribedil sustained-release tablets, 10% skeleton material, 25% lactose, and 25% microcrystalline cellulose. The skeleton material is a mixture of hydroxypropylmethylcellulose K4M, hydroxypropylmethylcellulose K15M and acrylic resin RS PO; by mass ratio, hydroxypropylmethylcellulose K4M: hydroxypropylmethylcellulose K15M: acrylic resin RS PO is 2:1:1, and the acrylic resin RS PO is a copolymer of ethyl acrylate, methyl methacrylate and trimethylaminoethyl methacrylate chloride, and the molar ratio of the three is 1:2 : 0.1. Then use 1% ethanol with a concentration of 50% as a binder to make a soft material, granulate with a 18-mesh sieve, dry at 60°C for 2 hours, and after granulation with a 18-mesh sieve, add 0.5% magnesium stearate, mix well, and press piece.
Embodiment 2
[0034] Embodiment 2: Preparation of piribedil sustained-release tablets
[0035] Prepare 30g piribedil sustained-release tablets, first take by weighing piribedil that accounts for 20% of the quality of piribedil sustained-release tablets, 28.5% skeleton material, 25% lactose, and 23% microcrystalline cellulose. The skeleton material is a mixture of hydroxypropylmethylcellulose K4M, hydroxypropylmethylcellulose K15M and acrylic resin RS PO; by mass ratio, hydroxypropylmethylcellulose K4M: hydroxypropylmethylcellulose K15M: acrylic resin RS PO is 2:1:1, and the acrylic resin RS PO is a copolymer of ethyl acrylate, methyl methacrylate and trimethylaminoethyl methacrylate chloride, and the molar ratio of the three is 1:2 : 0.1. Then use 3% ethanol with a concentration of 50% as a binder to make a soft material, granulate with a 18-mesh sieve, dry at 60°C for 2 hours, and after granulation with a 18-mesh sieve, add 0.5% magnesium stearate, mix well, and press piece.
Embodiment 3
[0036] Embodiment 3: Preparation of piribedil sustained-release tablets
[0037] Prepare 30g piribedil sustained-release tablets, first take by weighing piribedil that accounts for 20% of the quality of piribedil sustained-release tablets, 38.5% skeleton material, 20% lactose, and 20% microcrystalline cellulose. The skeleton material is a mixture of hydroxypropylmethylcellulose K4M, hydroxypropylmethylcellulose K15M and acrylic resin RS PO; by mass ratio, hydroxypropylmethylcellulose K4M: hydroxypropylmethylcellulose K15M: acrylic resin RS PO is 2:1:1, and the acrylic resin RS PO is a copolymer of ethyl acrylate, methyl methacrylate and trimethylaminoethyl methacrylate chloride, and the molar ratio of the three is 1:2 : 0.1. Then use 1% ethanol with a concentration of 50% as a binder to make a soft material, granulate with a 18-mesh sieve, dry at 60°C for 2 hours, and after granulation with a 18-mesh sieve, add 0.5% magnesium stearate, mix well, and press piece.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com