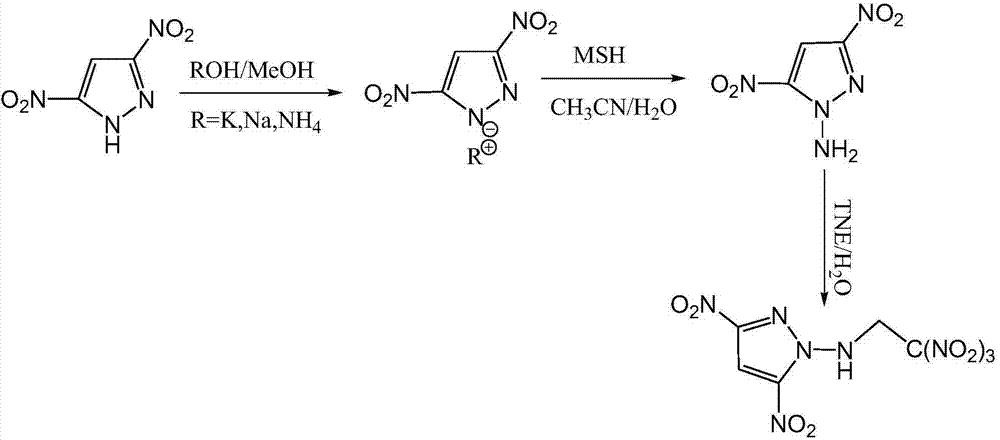
Energetic compound 1-(2,2,2-trinitroethylamino)-3,5-dinitropyrazole and preparation method thereof
A technology of trinitroethylamine and dinitropyrazole, which is applied in the field of energy-containing compounds 1--3,5-dinitropyrazole and its preparation, and can solve the problems of corrosion of metal materials, low energy content, etc. problems, to achieve the effect of less corrosiveness, mild reaction conditions and fewer reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Dissolve 3,5-dinitro-1H-pyrazole (10mmol, 1.58g) in methanol (50mL), add potassium hydroxide (10mmol, 0.56g), heat to reflux, stir for 2h, and filter out the solid. 3,5-Dinitropyrazole-N-potassium salt is obtained. In an ice-water bath, the prepared 3,5-dinitropyrazole-N-potassium salt was dissolved in 30 mL of N,N-dimethylformamide (DMF), and then MSH (20 mmol, 4.3 g) was dissolved in DMF (20 mL) was configured into a solution, slowly added dropwise to the above potassium salt solution, returned to room temperature and stirred for 12 hours. The solvent was spin-dried, ethyl acetate was added, and a white solid was precipitated. It was filtered off, and the filtrate was distilled under reduced pressure to obtain a yellow oil. A small amount of distilled water was added to precipitate a yellow solid, which was filtered off to obtain 1.08 g of N-amino-3,5-dinitropyrazole, with a yield of 62.4%. m.p.110°C, T d :264℃, MS(EI): m / z 172.01[M-H] - .
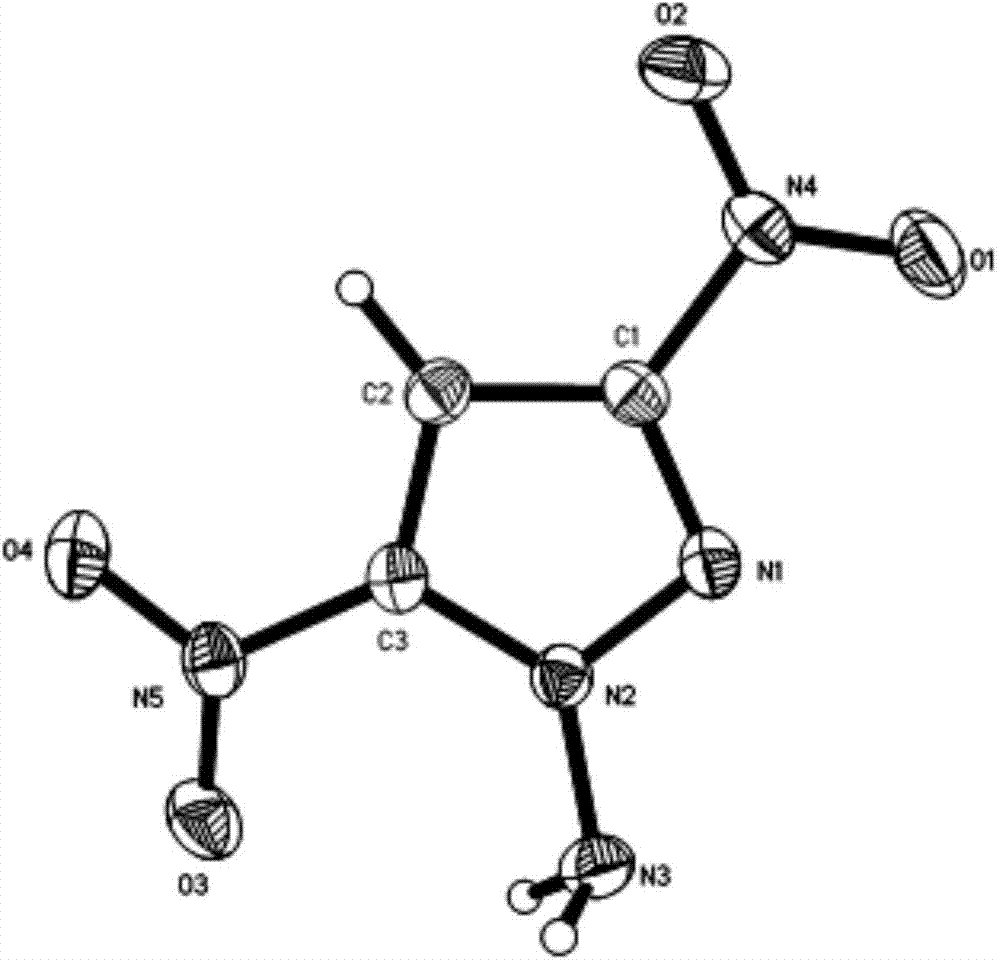
[0036] figure 2 It...
Embodiment 2
[0039]Dissolve 3,5-dinitro-1H-pyrazole (10mmol, 1.58g) in methanol (50mL), add sodium hydroxide (10mmol, 0.40g), heat to reflux, stir for 2h, and filter out the solid. 3,5-Dinitropyrazole-N-sodium salt is obtained. In an ice-water bath, the above sodium salt was dissolved in DMF (30 mL), and then MSH (20 mmol, 4.3 g) was dissolved in DMF (20 mL) to form a solution, which was slowly added dropwise to the potassium salt solution, returned to room temperature and stirred for 12 hours. The solvent was spin-dried, ethyl acetate was added, and a white solid was precipitated. It was filtered off, and the filtrate was distilled under reduced pressure to obtain a yellow oil. A small amount of distilled water was added to precipitate a yellow solid, which was filtered off to obtain 1.05 g of N-amino-3,5-dinitropyrazole, with a yield of 60.7%. Add N-amino-3,5-dinitropyrazole (1mmol, 173mg) into distilled water (30mL), heat to 80°C for 1h under stirring, then cool down to 50°C, slowly a...
Embodiment 3
[0041] 3,5-Dinitro-1H-pyrazole (10mmol, 1.58g) was added to excess concentrated ammonia water (100mL, mass concentration 22%), stirred at room temperature for 2h, and the solvent was spin-dried to obtain 3,5-dinitro Pyrazole-N-ammonium salt. In an ice-water bath, the ammonium salt was dissolved in DMF (30 mL), and then MSH (20 mmol, 4.3 g) was dissolved in DMF (20 mL) to form a solution, which was slowly added dropwise to the potassium salt solution, returned to room temperature and stirred for 12 hours. The solvent was spin-dried, ethyl acetate was added, and a white solid was precipitated. It was filtered off, and the filtrate was distilled under reduced pressure to obtain a yellow oil. A small amount of distilled water was added to precipitate a yellow solid, which was filtered off to obtain 1.12 g of N-amino-3,5-dinitropyrazole, with a yield of 64.8%. Add N-amino-3,5-dinitropyrazole (1mmol, 173mg) into distilled water (30mL), heat to 80°C for 1h under stirring, then cool...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com