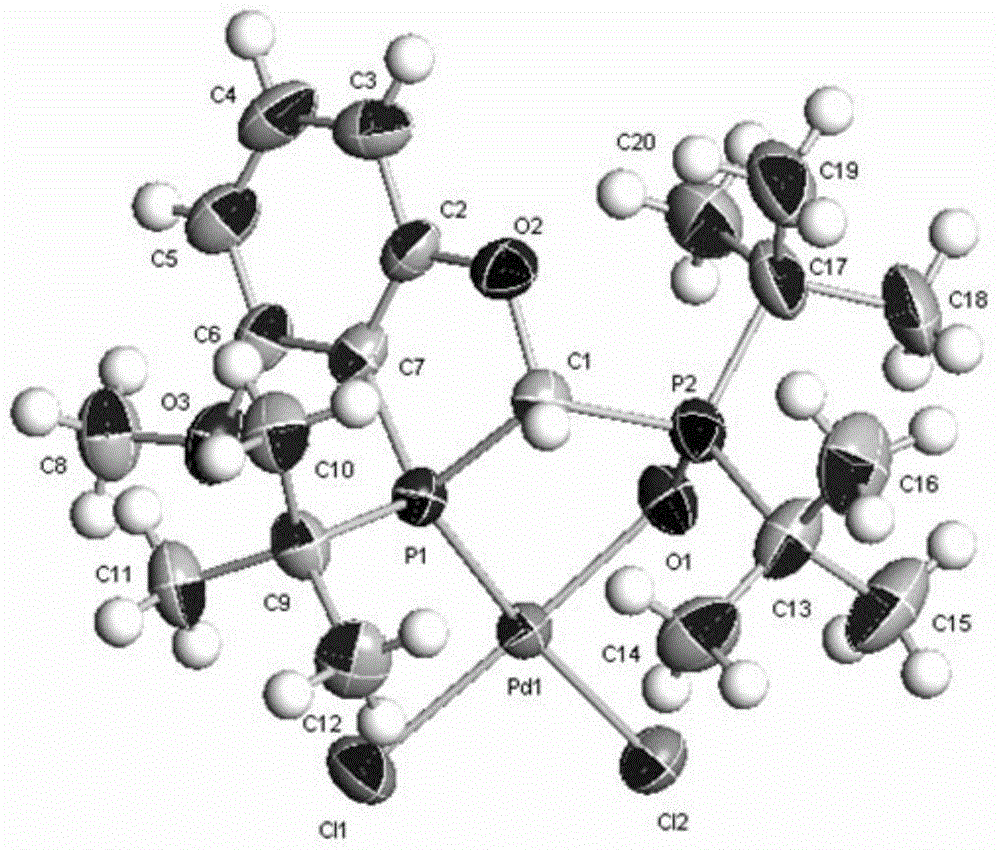
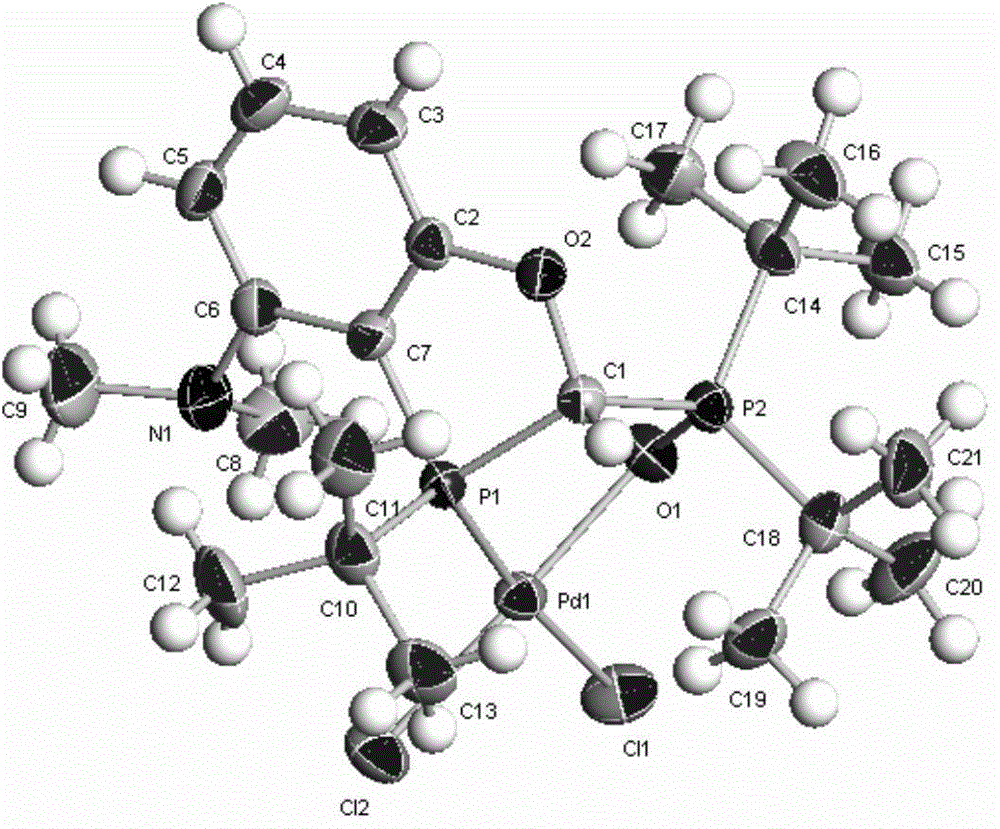
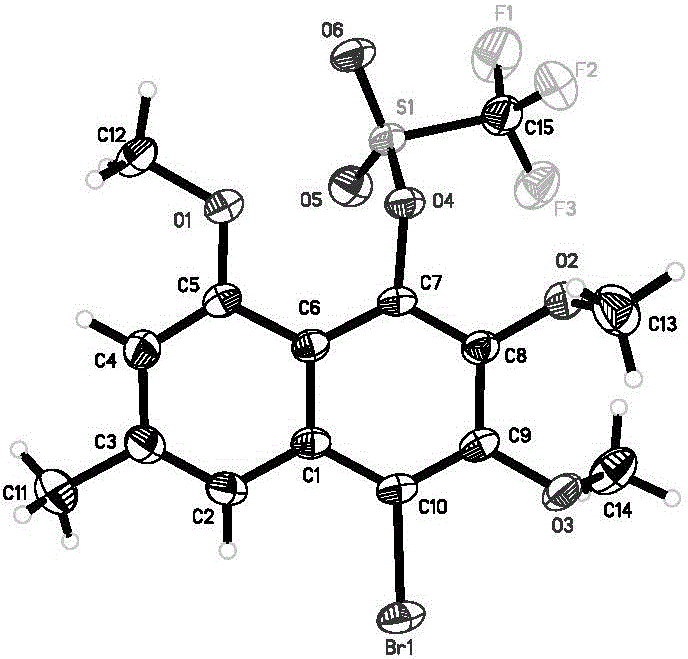
Chiral phosphorous ligand as well as metal catalyst containing ligand and application of chiral phosphorous ligand and catalyst
A technology of ligand compounds and transition metals, applied in the field of gossypol synthesis, can solve the problems of poor reaction selectivity and low yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0216] Preparation method of bidentate phosphine-phosphine oxygen ligand compound
[0217] The present invention also provides a preparation method of the bidentate phosphine-phosphine oxygen ligand compound shown in formula I,
[0218]
[0219] The method comprises the steps of:
[0220] (a) in an organic solvent, the compound of formula e is selectively reduced to a single phosphine-oxygen double bond, thereby forming a bidentate phosphine-phosphine-oxygen ligand compound shown in formula I,
[0221]
[0222] In each formula, R, R' and R" are as defined above.
[0223] Typically, the organic solvent in step (a) includes (but not limited to): toluene, tetrahydrofuran, anhydrous diethyl ether, or a combination thereof.
[0224] In another preferred embodiment, the reaction temperature in step (a) is 40-100°C, preferably 50-90°C, more preferably 60-90°C.
[0225] In another preferred embodiment, the reaction time of step (a) is 3-48 hours, preferably 5-24 hours, more p...
Embodiment 1
[0328] Preparation of bidentate phosphine-phosphine oxygen ligand compound L4
[0329]
[0330] 1. Synthesis of compound b
[0331] Compound a (1.23g, 8.15mmol, 1.1equiv) was dissolved in 15mL of tetrahydrofuran, cooled to 0°C, n-butyllithium (3.20mL, 8.15mmol, 1.1equiv) was slowly added dropwise, and stirred at 0°C for 1 hour. Then slowly add tert-butylphosphorous dichloride (1.18g, 7.41mmol, 1.0equiv) dissolved in tetrahydrofuran (15mL) to the reaction system at 0°C, move the reaction system to room temperature and stir for 4 hours before adding 3mL water, reacted at room temperature for 3 hours. At room temperature, sodium hydroxide solution (2M, 7.4mL, 14.82mmol, 2.0equiv) and formaldehyde solution (37%, 1.60mL, 3.0equiv) were added dropwise to the reaction system to ensure that the temperature of the system was always lower than 30°C. Afterwards, the reaction system was stirred at room temperature for 3 hours. Add dilute hydrochloric acid (6M) to the reaction system...
Embodiment 2
[0346] Preparation of bidentate phosphine-phosphine oxygen ligand compound L3
[0347]
[0348] 1. Synthesis of Ligand L3
[0349] Compound f (0.38 g, 1.00 mmol, 1.0 equiv) was put into a Schlenk tube, and toluene (5 mL) was added after pumping nitrogen three times. Add HSiCl to this reaction system 3 (0.51 ml, 5.00 mmol, 5.0 equiv) and TEA (1.40 mL, 10.00 mmol, 10.0 equiv). The reaction system was stirred at 80° C. for 12 hours, cooled to room temperature, and the toluene was vacuum-dried. Add 30% sodium hydroxide aqueous solution (20mL) dropwise to the reaction system, hydrogen gas is generated during the dropwise addition, the reaction system is heated and stirred at 60°C for 30 minutes and then cooled to room temperature, and MTBE (20mL×3 ) extraction, the combined organic phases were added to dry over anhydrous sodium sulfate. Filtered and dried by pumping through a neutral alumina column to obtain white solid L3 (0.35g, 0.91mmol, 96%).
[0350] Ligand L3: 1 H NM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com