Synthetic method for MDJ (Methyl Dihydrojasmonate)
A technique for the synthesis of methyl dihydrojasmonate, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, can solve problems such as ineffective effects, and achieve non-corrosiveness and reaction routes Simple, cheap ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
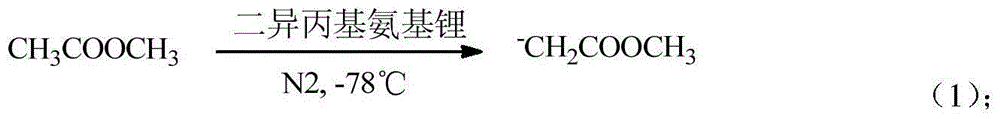
[0018] Into a three-necked flask equipped with a stirrer, a thermometer, and a dropping funnel, N 2 Protection, cool down to -78°C with dry ice and keep warm. After the temperature in the three-neck flask is stabilized at -78°C, start adding 18.5g of methyl acetate, then add 5ml of LDA with a syringe under stirring, and continue stirring to make lithium diisopropylamide Dissolve until the solution changes from colorless to light yellow, which is the generation of active methylene ions.
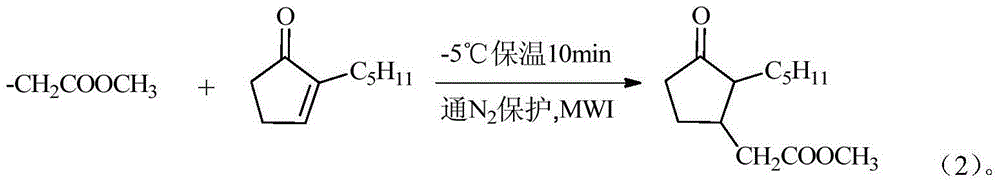
[0019] Change the ice-salt bath to adjust the temperature to -5°C, then add 38g of lactone dropwise to the dropping funnel, after 0.5h of dropwise addition, stir the reaction for 10min under the microwave power of 0.5KW to terminate the reaction, add dilute HCl to adjust pH to 6-7, then washed with saturated brine and left to separate layers, washed 3 times, collected the upper organic phase, evaporated the solvent and water under normal pressure, rectified under reduced pressure, and collecte...
Embodiment 2
[0021] Into a three-necked flask equipped with a stirrer, a thermometer, and a dropping funnel, N 2 Protection, cool down to -78°C with dry ice and keep warm. After the temperature in the three-necked flask is stabilized at -78°C, start adding 18.5g of methyl acetate, then add 10ml of LDA with a syringe under stirring, and continue stirring to make lithium diisopropylamide Dissolve until the solution changes from colorless to light yellow, which is the generation of active methylene ions.
[0022] Change the ice-salt bath to adjust the temperature to -5°C, then add 31.65g of internal ketone to the dropping funnel, after 0.5h of dropwise addition, stir the reaction for 10min under the microwave power of 0.5KW to terminate the reaction, add dilute HCl to adjust pH to 6-7, then wash with saturated brine and let stand to separate layers, wash 2-3 times, collect the upper organic phase, distill off the solvent and water under normal pressure, rectify under reduced pressure, and col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com