Tumor stroma pH sensitive target dendrimer and preparation method thereof
A dendritic polymer and tumor stroma technology, applied in the field of medicine, can solve problems such as toxic side effects, blood cell destruction, application limitations, etc., and achieve the effect of reducing toxic side effects and promoting transport
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Preparation of pH-sensitive targeting dendrimers
[0017] Step 1, preparation of CMCS: Dissolve 1 g of chitosan in 15 mL of 50% wt NaOH solution, alkalinize for 24 h and then filter with suction, add the obtained solid I to 2.5 times the weight of chloroacetic acid, and react at room temperature for 24 h After suction filtration, add 50 mL of water to dissolve the obtained solid II, adjust the pH of the solution to 7.0 with 2M HCl, centrifuge the solution, add 100 mL absolute ethanol to the supernatant, suction filter, wash the obtained solid III with absolute ethanol, and The solid III was dried at 30°C, dissolved in 10 mL of water, dialyzed in water to remove impurities with a dialysis bag with a molecular weight of 3500, and freeze-dried for 72 hours to obtain 0.89 g of a white product.
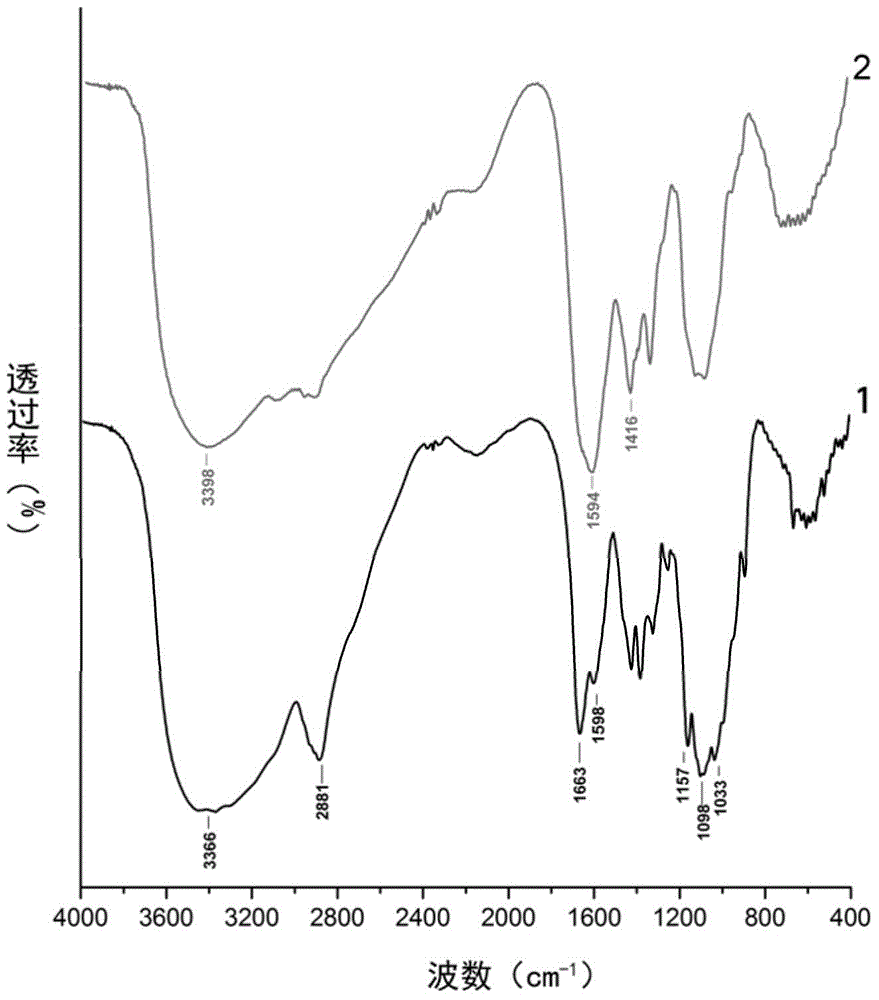
[0018] figure 1 For the selected chitosan of embodiment 1 and the infrared spectrogram of the carboxymethyl chitosan of preparation. Infrared spectrum analysis shows that the main...
Embodiment 2
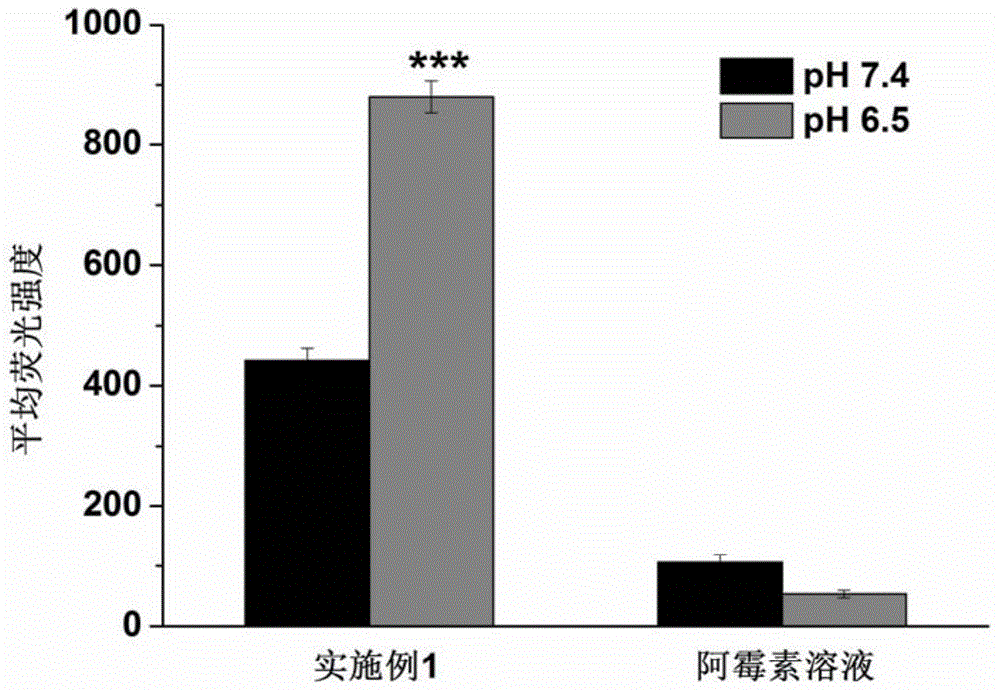
[0024] In vitro cellular uptake assay
[0025] Using the commercially available doxorubicin solution as a control, an in vitro cell uptake test was carried out to investigate the targeting and ability of the pH-sensitive targeting dendrimer obtained in Example 1 to promote cell uptake.
[0026] Human breast cancer MCF-7 cells were cultured at a density of 1×10 5 Cells / well were seeded in 24-well plates and cultured for 24 hours. Thereafter, cells were treated with fresh medium (pH 6.5 and pH 7.4) containing samples (doxorubicin solution and targeting dendrimer obtained in Example 1, the same final doxorubicin concentration was 6.95 μg / mL) for 4 h (37°C). After the cells were incubated for 4 h, they were washed 3 times with fresh PBS, and then the cells were digested with trypsin. After discarding the trypsin, the cells were made into a cell suspension with PBS. After centrifugation at 1000 r / min for 5 min, the supernatant was discarded, and 200 Disperse the cells in μL PBS,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com