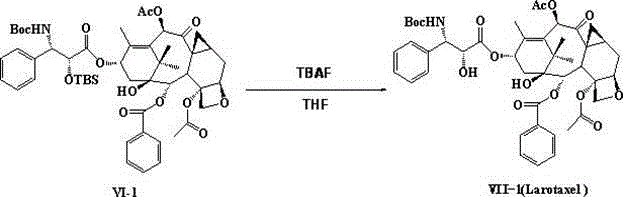
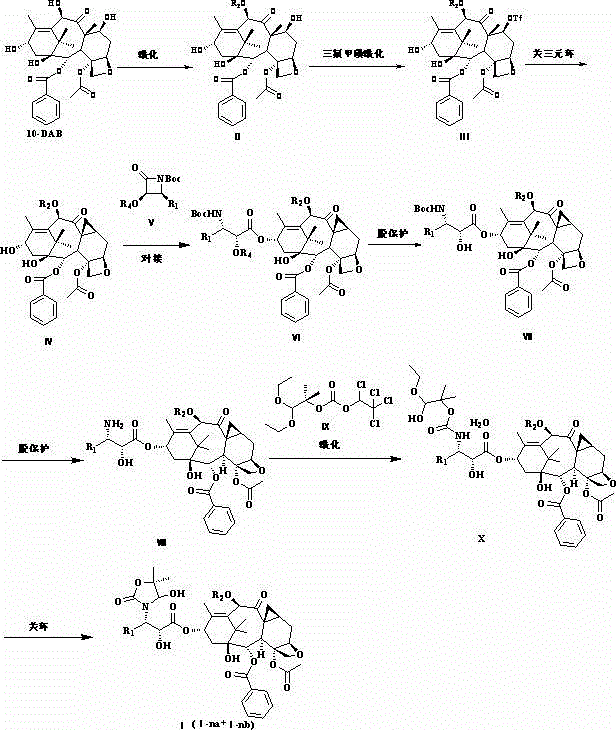
Semi-synthetic taxane derivative as well as preparation method and application thereof
A technology for taxanes and derivatives, applied in the field of semi-synthetic taxane derivatives and their preparation and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Preparation of compound II-1.
[0053]
[0054] Will 10-DAB (54.5g, 100.0mmol) was dissolved in dry tetrahydrofuran (1L), under argon protection, acetic anhydride (100.0mL, 1.0mol) and cerium trichloride heptahydrate (1.86g, 5.0mmol) were added under ice-cooling, slowly Return to room temperature, stir and react for 3 h, after the completion of the reaction as detected by thin-layer chromatography, the reaction solution was concentrated and evaporated to dryness, the resultant was diluted with ethyl acetate (1.5L), and distilled water (500mL×3) and saturated aqueous sodium chloride ( 500mL×3), washed with anhydrous sodium sulfate, concentrated to obtain a white solid crude product, and recrystallized to obtain 56.0g of white solid product II-1, with a yield of 96.0%.
[0055] 1 H NMR (600 MHz, CDCl 3 ) δ 8.10 (d, J = 7.2 Hz, 2H), 7.61 (t, J = 7.8 Hz, 1H), 7.48 (t, J =7.2 Hz, 2H), 6.32 (s, 1H), 5.62 (d, J = 7.2 Hz, 1H), 4.99 (d, J = 8.4 Hz, 1...
Embodiment 2
[0056] Example 2 Preparation of Compound III-1.
[0057]
[0058] Compound II-1 (51.5g, 88.0mmol) was dissolved in dry dichloromethane (1L), under argon protection, dry pyridine (200.0mL, 2.2mol) was added, the reaction solution was cooled to -35°C, and slowly Add trifluoromethanesulfonic anhydride (35.5mL, 220.0mmol) dropwise, control the dropwise addition within 1h, continue to stir for 4h, slowly return to room temperature and stir overnight, after the reaction is detected by thin layer chromatography, the reaction solution is dichloromethane (2.5L), washed successively with 1M aqueous sodium bisulfate solution (750mL×3), saturated aqueous sodium bicarbonate solution (300mL×3), saturated aqueous sodium chloride solution (300mL×3), dried over anhydrous sodium sulfate, and concentrated . The obtained orange-red solid crude product was dissolved in ethyl acetate / dichloromethane=1:2, petroleum ether was added until a large amount of solids were precipitated, left standing a...
Embodiment 3
[0060] Example 3 Preparation of compound IV-1.
[0061]
[0062] Compound III-1 (50.0g, 70.0mmol), sodium chloride (80.0g) and 4? molecular sieves (30.0g) were dissolved in dry acetonitrile / tetrahydrofuran=(1000mL / 100mL), under the protection of argon, prior to Stir at room temperature for 1 h, raise the temperature to 75°C and continue to stir for 2 h. After the reaction is detected by thin-layer chromatography, filter the reaction solution with suction, rinse the filter cake with a large amount of ethyl acetate, evaporate the filtrate to dryness, and wash with ethyl acetate (1.5 L ) was dissolved, washed successively with saturated aqueous sodium bicarbonate solution (300mL×3), saturated aqueous sodium chloride solution (300mL×3), dried over anhydrous sodium sulfate, concentrated, and subjected to column chromatography (dichloromethane / ethyl acetate=10 : 1) obtain white solid product IV-1 36.9g, yield 91.0%.
[0063] 1 H NMR (600 MHz, CDCl 3 ) δ 8.13 (d, J = 7.8 Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com