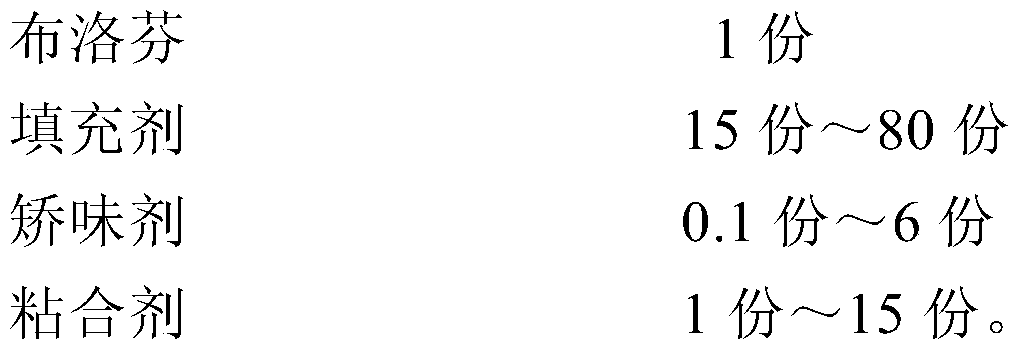Ibuprofen granule and preparation method thereof
A granule, ethanol aqueous solution technology, applied in pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. It is easy to deliquescence and inconvenient for children to use medicine, so as to achieve the effect of being suitable for industrial production, improving taste and pungent odor, and improving medication compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Pass the ibuprofen through a 100-mesh sieve, crush the sucrose, hypromellose K4M, sodium cyclamate and stevioside and pass through a 80-mesh sieve for later use.
[0036] Weigh the raw materials of the following prescription quantities:
[0037]
[0038] (1) Dissolve 6.1kg sucrose and 6.1kg sodium cyclamate in 100kg boiling water, stir well, and be mixed with filler aqueous solution; Hypromellose K4M is dissolved in 50% (volume percentage content) ethanol aqueous solution, stir Evenly, prepare 4.5 kg of 1% hypromellose K4M 50% ethanol aqueous solution, and set aside;
[0039] (2) Get ibuprofen 1.70kg and join in the coating granulator, start the main engine, set the rotating speed as 110 rev / min, spray the filler aqueous solution and 1.5kg 1% hypromellose of step (1) gained simultaneously K4M50% ethanol aqueous solution, peristaltic pump speed 15 rpm, after the granulator is dust-free, open the machine cover to observe and slow down the spray speed to 10 rpm, until ...
Embodiment 2
[0045] Pass the ibuprofen through a 100-mesh sieve, crush sucrose, polyvinylpyrrolidone, etc., and pass through an 80-mesh sieve for later use.
[0046] Weigh the raw materials of the following prescription quantities:
[0047]
[0048] (1) Dissolve 5.1kg of sucrose and 10.1kg of sodium cyclamate in boiling water, stir evenly, and prepare an aqueous filler solution; dissolve polyvinylpyrrolidone in 50% ethanol aqueous solution, stir evenly, and prepare 3% polyvinylpyrrolidone 5.1kg of 50% ethanol aqueous solution, for later use;
[0049](2) Get 2.50kg of ibuprofen and add it to the coating granulator, start the main engine, set the rotating speed to be 110 rpm, spray the aqueous filler solution and polyvinylpyrrolidine ethanol aqueous solution obtained in step (1) simultaneously, and the rotating speed of the peristaltic pump 15 rpm, after the granulator is free of dust, open the machine cover to observe and slow down the spray speed to 10 rpm, until the masterbatch is pro...
Embodiment 3
[0055] The ibuprofen is passed through a 100-mesh sieve, and the sucrose, polyvinylpyrrolidone, etc. are crushed and passed through a 80-mesh sieve for later use.
[0056] Weigh the raw materials of the following prescription quantities:
[0057]
[0058] (1) 10.1kg sucrose and 5.1kg sodium cyclamate were dissolved in boiling water, stirred evenly, and dissolved to be prepared as filler aqueous solution; polyvinylpyrrolidone was dissolved in ethanol aqueous solution and stirred uniformly, and 5.7kg of polyvinylpyrrolidone aqueous solution was prepared, spare;
[0059] (2) Get 3.50 kg of ibuprofen and add it to the coating granulator, start the main engine, set the rotating speed at 110 rpm, spray the aqueous filler solution obtained in step (1) and the ethanol aqueous solution of polyvinylpyrrolidone at the same time, and the rotating speed of the peristaltic pump is 15 After the granulator is clean, open the cover to observe and slow down the spray speed to 10 rpm until t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com