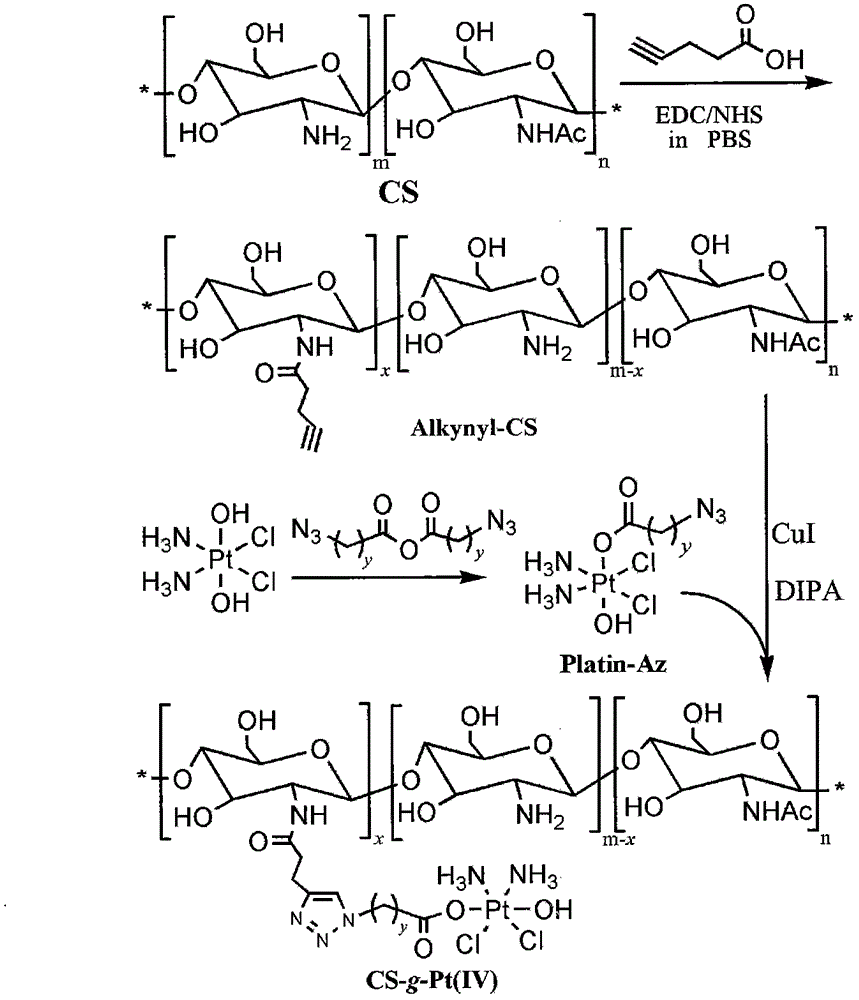
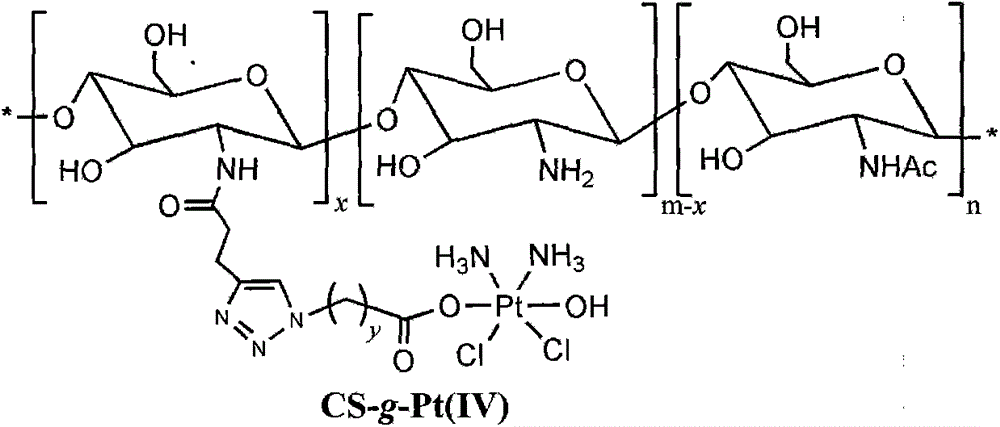
Chitosan-platinum (IV) prodrug conjugate and preparation method thereof
A prodrug, chitosan technology, used in drug combinations, pharmaceutical formulations, antitumor drugs, etc., can solve problems such as drug concentration fluctuations, toxic side effects, etc., to enhance tissue effects, reduce toxic side effects, and improve blood circulation time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1 Chitosan-platinum (IV) prodrug conjugate
[0027] 1) Synthesis of 4-pentynoic acid modified chitosan (alkynyl-CS)
[0028] Under the action of the combined catalyst, the amino group of chitosan and the carboxyl group of 4-pentynoic acid form an amide bond through amide chemical reaction to obtain alkynyl functionalized chitosan:
[0029] Weigh chitosan (degree of deacetylation 89%, M w =10kDa) 0.5g (2.73mmol amino) and 0.268g (2.73mmol) of 4-pentynoic acid are dissolved in 50mL 0.1M PBS (pH=5) solution, degassed, protected by nitrogen, and then slowly added EDC 0.55 g (2.73mmol) and NHS 0.32g (2.73mmol). After stirring and reacting at room temperature for 18 hours, the reaction solution was transferred to a dialysis bag with a molecular weight cut-off of 3000Da, and then dialyzed with double distilled water for 48 hours at low temperature, and then lyophilized to obtain 0.55g Target product (87%).
[0030] 2) Synthesis of azide anhydride functionalized platinum (IV) ...
Embodiment 2
[0034] Example 2 Chitosan-platinum (IV) prodrug conjugate
[0035] 1) Synthesis of 4-pentynoic acid modified chitosan (alkynyl-CS)
[0036] Under the action of the combined catalyst, the amino group of chitosan and the carboxyl group of 4-pentynoic acid form an amide bond through amide chemical reaction to obtain alkynyl functionalized chitosan:
[0037] Weigh chitosan (degree of deacetylation 89%, M w =10kDa) 1.0g (5.5mmol amino) and 0.73g (8.2mmol) of 4-pentynoic acid are dissolved in 50mL 0.1M PBS (pH=6) solution, degassed, protected by nitrogen, and then slowly added EDC 1.7 g (8.2mmol) and NHS1.0g (8.2mmol). After stirring and reacting at room temperature for 20 hours, the reaction solution was transferred to a dialysis bag with a molecular weight cut-off of 3000 Da, dialyzed with double distilled water at low temperature for 48 hours, and lyophilized to obtain 0.97 g Target product (83%).
[0038] 2) Synthesis of azide anhydride functionalized platinum (IV) prodrug
[0039] Weig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com