Method for fermentation production of L-proline by utilizing recombinant Escherichia coli
A technology of Escherichia coli and proline, applied in the direction of fermentation, recombinant DNA technology, bacteria, etc., to achieve the effect of broad industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Acquisition of glutamate kinase gene
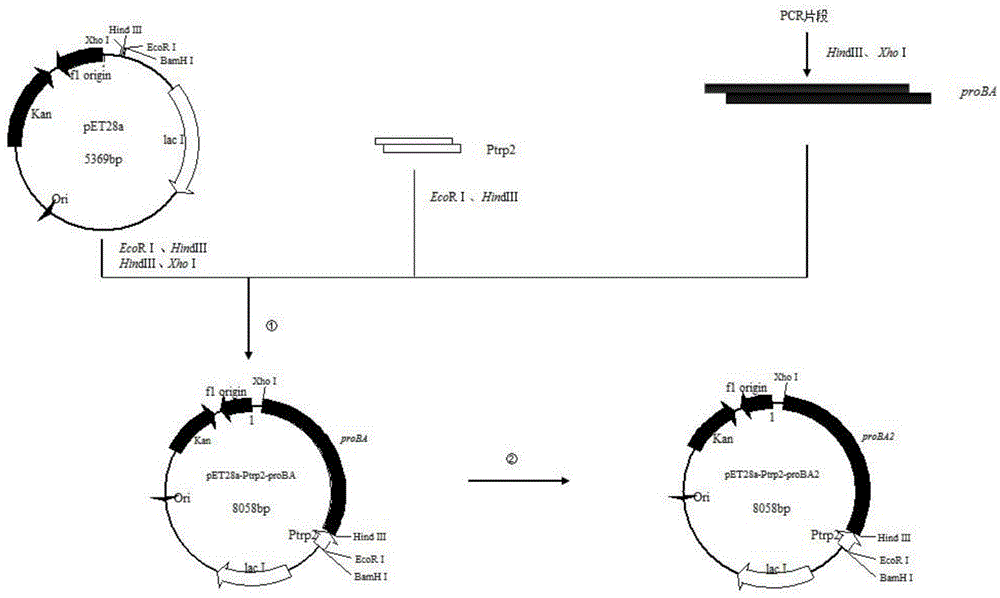
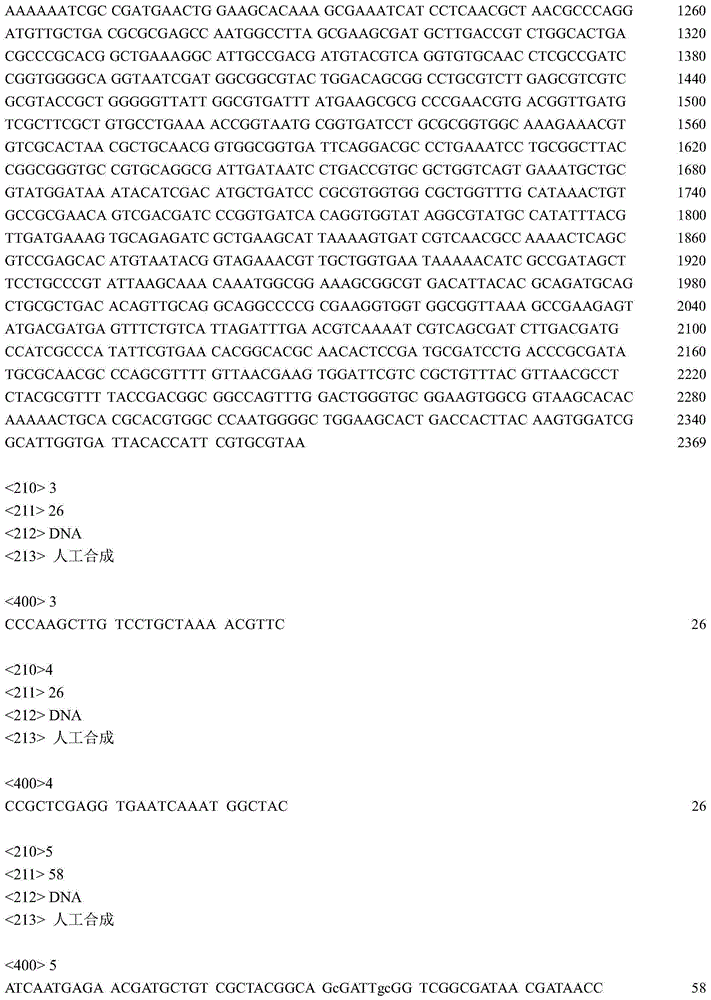
[0025] In the synthesis of proline, glutamate kinase (proB) and glutamate dehydrogenase (proA) always complement each other, the expression and effect of the two will affect each other, and the genes of the two are also close to each other, so In this embodiment, the two are designed as a whole to obtain the proBA gene fragment, and a pair of primers P1 (SEQ ID NO: 3), P2 (SEQ ID NO: 3) and P2 (SEQ ID NO: 3) are designed according to the proBA gene sequence published in NCBI (GenBank accession number: x00786. ID NO: 4), the target gene proBA was obtained by PCR amplification.
[0026] In the present embodiment, the template of PCR is Escherichia coli genome, extraction method: get 1.5mL bacterium liquid (overnight culture), 12000rpm centrifuges 30s to collect thalline; Under the situation that does not affect bacterial precipitation, remove supernatant as far as possible; Thalline Resuspend the pellet in 600 μL lysate (...
Embodiment 2
[0027] Example 2: Acquisition of tryptophan tandem promoter gene
[0028] The tryptophan tandem promoter is derived from the tryptophan operon of Escherichia coli K12 strain, and is a strong promoter suitable for industrial production applications. Since two tryptophan tandem promoters can be connected in series to increase the expression intensity, other researchers in the laboratory optimized the tryptophan tandem promoter and then synthesized the tryptophan tandem promoter in the whole gene.
[0029] See SEQ ID NO:1 for the sequence of the tryptophan tandem promoter in this embodiment.
Embodiment 3
[0030] Embodiment 3: the construction of proBA expression plasmid and recombinant bacteria
[0031] First obtain the proBA gene, the proBA gene needs to be amplified by PCR, and the obtained proBA gene 5' end sequence has a HindⅢ (A^AGCTT) restriction site, and the 3' end has an XhoI (C^TCGAG) restriction site , the proBA gene and plasmid vector pET-28a were digested with HindⅢ and XhoI respectively, and the proBA gene and pET-28a plasmid vector after the double-cut enzyme were recovered with an agarose gel kit, and then proBA and pET were digested with T4 DNA ligase. -28a gene fragments were connected. After overnight ligation at 16°C, transfer 10 μL of the ligation solution into Escherichia coli JM109. Pick the transformed single colony culture to extract the plasmid for verification, and then further sequence to verify whether the gene sequence is correct. Thus, the constructed pET-28a-proBA recombinant plasmid was obtained, and the recombinant Escherichia coli JM109 / pET-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com