Telmisartan amorphous crystal and preparation method thereof
A technology of telmisartan and crystal form, which is applied in the field of pharmaceutical engineering and cardiovascular disease treatment drugs, can solve the problems of difficulty in obtaining telmisartan amorphous crystal form, small amount of drying, long cycle, etc., and achieve easy industrialization The effect of production, good dissolution rate and safe preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
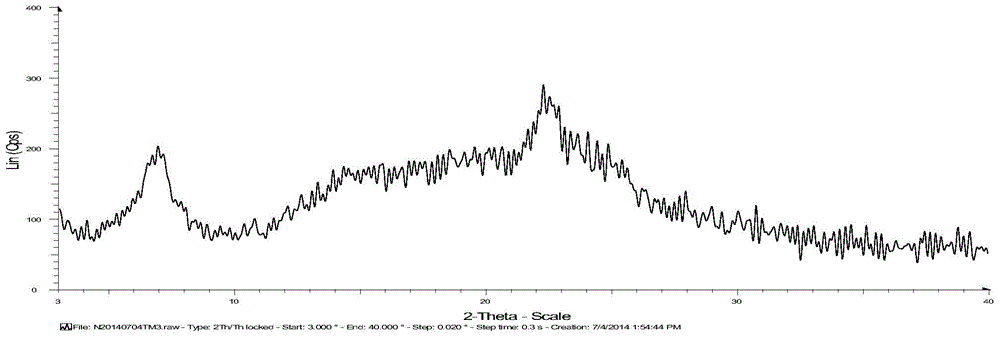
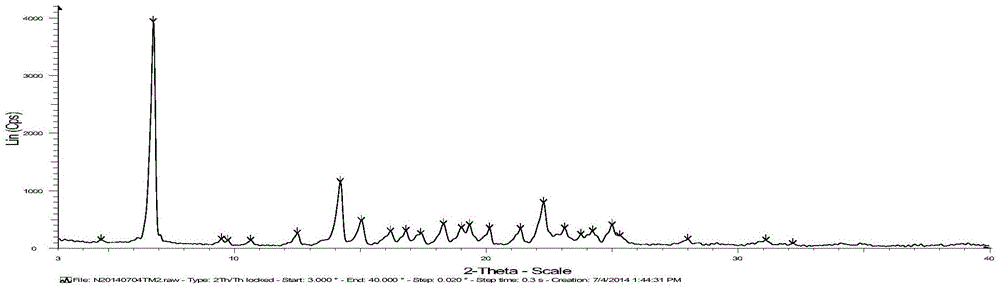
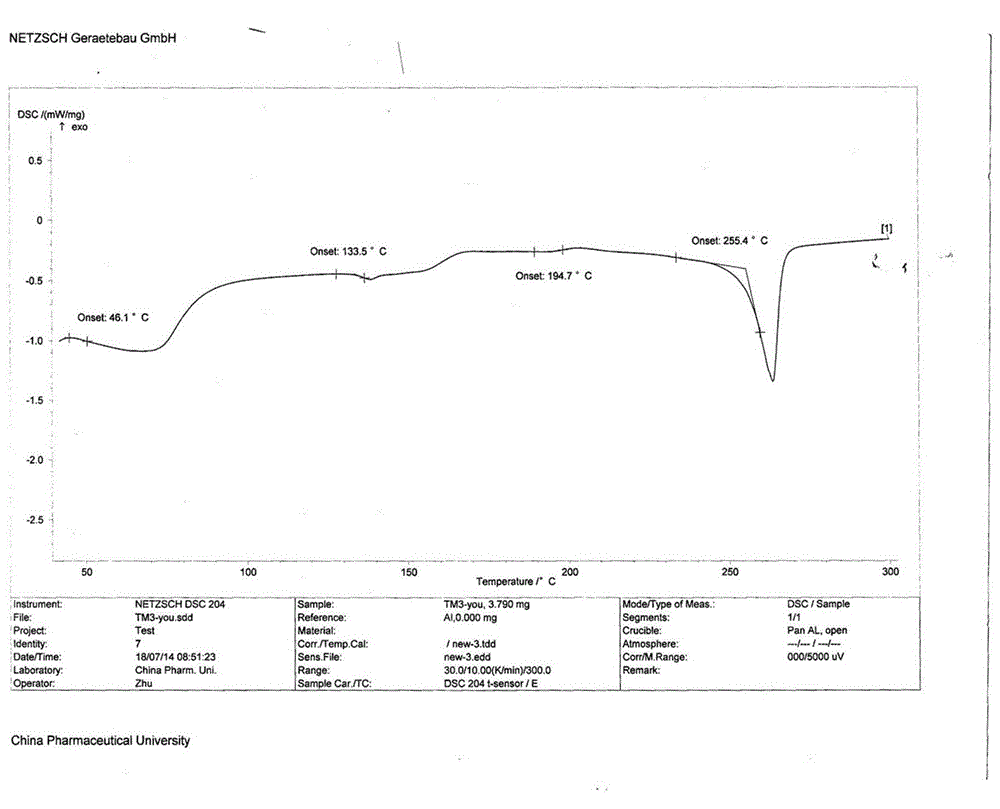
[0042] Add 38.9kg of crude telmisartan to a 1000L crystallization kettle, pump in 110kg of methanol, pump in 21.6kg of ammonia water at room temperature, heat to 75-85°C and reflux until the solid is completely dissolved, filter the insoluble matter while the system is hot, and evaporate the solvent , add 116.7kg of water to the system, add 11-12kg of acetic acid in batches at room temperature to adjust the pH to 5-6 (the amount of acetic acid is adjusted to the pH value of 5-6), after acidification is completed, centrifuge, and wash with purified water to pH 6-7, the material was taken out, and vacuum-dried at 80-125°C to obtain 37.12kg of telmisartan in amorphous crystal form, yield: 95.4%, XRD pattern of telmisartan in amorphous crystal form mp: 255.4°C.
[0043] 1HNMR(DMSO): δ=1.01(t,J=7.5Hz,3H,CH3); δ=1.82(m,2H,CH2); δ=2.63(s,3H,CH3); δ=2.93(t,J =7.5Hz, 2H, CH2); δ = 3.83 (s, 3H, CH3); δ = 5.63 (s, 2H, CH2); δ = 7.17-7.73 (m, 14H, CH). MS (EI): 514 (m+).
[0044] Its ...
Embodiment 2
[0054]Add 38.9kg of crude telmisartan to a 1000L crystallization kettle, pump in 116.7kg of ethanol, pour in a solution of 16kg of sodium carbonate and 20kg of drinking water prepared in advance at room temperature, heat to 75-85°C and reflux until the solid is completely dissolved , the system filtered the insoluble matter while it was hot, evaporated the solvent, added 116.7kg of water to the system, added 10% hydrochloric acid in batches at room temperature to adjust the pH to 5-6 (the consumption of hydrochloric acid is adjusted to 5-6 based on the pH value), After acidification, centrifuge, wash with purified water until the pH is 6-7, take out the material, and dry it in vacuum at 80-125° C. to obtain 30.9 kg of telmisartan in amorphous crystal form.
Embodiment 3
[0056] Add 38.9kg of crude telmisartan to a 500L crystallization kettle, pump in 77.8kg of ethanol, pour in a solution of 6.05kg of sodium hydroxide and 15kg of drinking water prepared in advance at room temperature, heat at 75-85°C and reflux until the solid is completely Dissolve, filter the insoluble matter while the system is hot, distill off the solvent, add 116.7kg of water to the system, cool the system to 0°C, and slowly add concentrated sulfuric acid to adjust the pH to 5-6 after the temperature stabilizes (the amount of concentrated sulfuric acid is determined by pH The value is adjusted to 5-6 as the criterion), acidification is completed, centrifuged, washed with purified water until the pH is 6-7, the material is taken out, and vacuum-dried at 80-125°C to obtain 32.8kg of telmisartan in amorphous crystal form.
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com