Copper coordination polymer having ion exchange and solvent exchange functions and application of copper coordination polymer
A technology of coordination polymers and copper complexes, applied in the field of copper coordination polymers and preparation, Cu-anthracycline bistriazole two-dimensional copper coordination polymers, can solve the problems of rare two-dimensional structure, and achieve the reaction The operation is simple and easy, the reaction yield is high, and the method is simple and effective
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of 1-[9-(1H-1,2,4-triazol-1-yl)anthracen-10-yl]-1H-1,2,4-triazole (tatrz)
[0031] The molar ratio of 9,10-dibromoanthracene: triazole: potassium carbonate: copper oxide is 2:10:30:1;
[0032] CuO (0.0398 mg, 0.5 mmol), potassium carbonate (2.0731 g, 15 mmol), triazole (0.345 mg, 5 mmol), and 9,10-Dibromoanthracene (0.3360 g, 1 mmol), 20 mL DMF. Start stirring at 100 o C, reacted for 24 hours. After the reaction, the reaction solution was lowered to room temperature, filtered, and 100 mL of water was added to the filtrate, a large amount of precipitate was precipitated, filtered with suction, and the filter cake was collected, with a yield of 60%.
Embodiment 2
[0034] Complex 1 preparation of
[0035] Cu(NO 3 ) 2 and 1-[9-(1H-1,2,4-triazol-1-yl)anthracen-10-yl]-1H-1,2,4-triazole (tatrz) in a molar ratio of 1: 1;
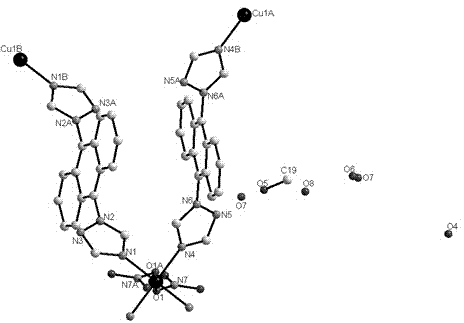
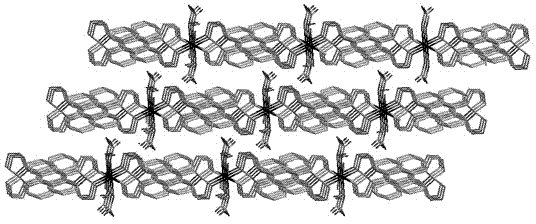
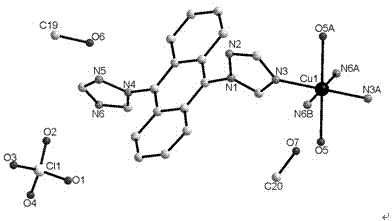
[0036] tatrz (0.0625 g, 0.2 mmol) and Cu(NO 3 ) 2 (0.0483 g, 0.2 mmol) in H 2 O (6 mL) and CH 3 OH (4 mL) in a mixed solvent at room temperature for half an hour and then filtered, and the filtrate was volatilized at room temperature for two weeks to obtain green rod-shaped crystals suitable for X-ray single crystal diffraction analysis. Yield: 69%. Elemental analysis (%) C 37 h 38 CuN 14 o 12 : C 47.56, H 4.10, N 20.99; Found: C 47.68, H 4.08, N 20.88.
Embodiment 3
[0038] Ion exchange properties of complexes
[0039] in the complex 1 (0.4672 g, 0.5 mmol) and NaClO 4 (0.7023 g, 5 mmol) in a mixed solvent of water and methanol (10 mL, v:v = 5:5) was stirred for 6 hours, and green rod-shaped crystals suitable for X-ray single crystal diffraction analysis were obtained 1a , Yield: 25%. Elemental Analysis (%) C 40 h 44 Cl 2 CuN 12 o 14 : C 45.70, H 4.22, N 15.99; Found: C 45.58, H 4.02, N 15.78.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com