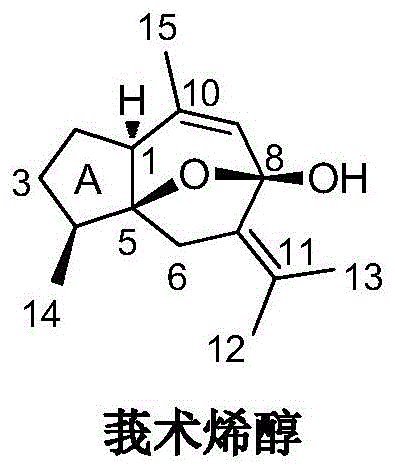
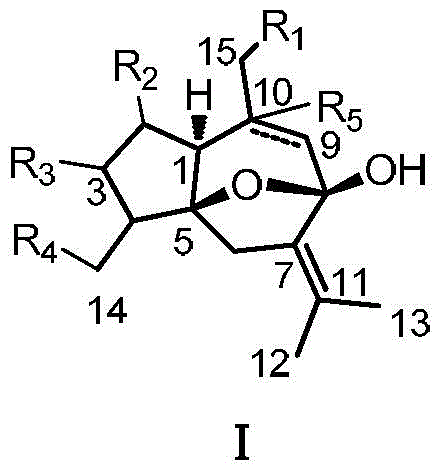
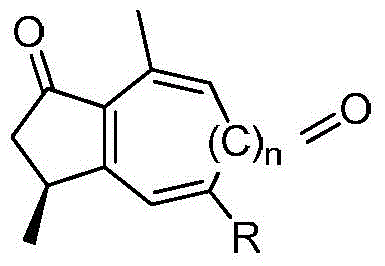
Curcumenol microbial transformation derivatives, preparation method and use thereof
A technology of curcumenol and hydroxycurcumenol, which is applied in the field of medicine to achieve the effects of increasing structural modification sites, preventing and treating inflammation, and increasing polarity and water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the microbial conversion of curcumenol and the separation of conversion product
[0031] The specific steps of microbial transformation are as follows:
[0032] a) The preparation of potato liquid culture medium: take fresh potato, peel, cut into 2cm 3 For small pieces, add 5 times the amount of water to boil, keep boiling for 30 minutes, filter the residue with gauze, add water to the filtrate (1 liter of culture medium per 200 grams of potatoes), and add 2% glucose after constant volume. Divide the prepared culture solution, 250mL Erlenmeyer flask can be filled with 60mL culture solution, and 500mL Erlenmeyer flask can be filled with 200mL culture solution. Seal the mouth of the bottle with gauze and kraft paper, and put it in an autoclave for sterilization. The sterilization condition is 115°C for 30 minutes.
[0033] b) Microbial cultivation: Three kinds of fungal strains Penicillium urticae IFFI 04015, Cunninghamella echinulata AS 3.3400, and Mucor...
Embodiment 2
[0067] Example 2: Inhibitory activity experiment of transformation products and substrates on release of nitric oxide (NO) from mouse mononuclear macrophage RAW 264.7 induced by lipopolysaccharide
[0068] Mouse mononuclear macrophage RAW 264.7 (ATCC TIB-71) was cultured in 10% heat-inactivated (56°C, 30min) fetal bovine serum (FBS), 100 U / mL penicillin sodium (Gibco), 100 μg / mL streptavidin in RPMI 1640 (Gibco) medium, 37°C, 5% CO 2 grown in a constant temperature incubator. Because NO is extremely unstable, it is quickly metabolized into nitrous acid (NO) in the cell culture supernatant. 2 - ), so the Griess method was used to measure NO in the sample 2 - concentration as an indicator of NO levels. Griess reagent A: 0.1% N-naphthalene ethylenediamine hydrochloride (naphthylene diaminedihydrochloride) dissolved in water: Griess reagent B: 1% p-aminobenzenesulfonamide (sulphanilamide) dissolved in 5% H 3 PO 4 middle. Mix reagents A and B in equal volumes before use. D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com